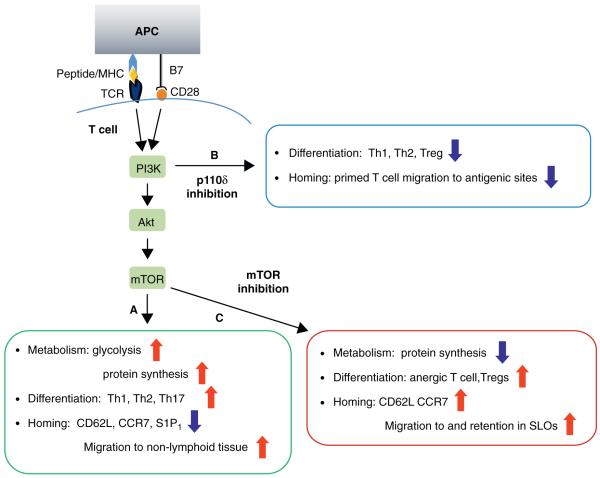
Figure 1.
Metabolic status regulates T cell activation, differentiation and homing. (A) Downstream of TCR and CD28, PI3K leads to activation of AKT, which subsequently controls mTOR activity. This signalling cascade promotes glucose metabolism and protein synthesis necessary for T cell activation, proliferation and differentiation into Th1, Th2 and Th17 subsets. The PI3K–AKT–mTOR axis also promotes downregulation of SLOs homing receptor CD62L, CCR7 and S1P1. Antigen experienced T cells, therefore, home to their respective non-lymphoid tissues. (B) Inhibition of PI3K via p110δ-selective inhibitor, IC87114, or genetic mutation reduces the capacity of T cells to differentiate along the Th1 and Th2 lineages. It also reduces the number and function of Tregs in peripheral organs, and compromises TCR-dependent migration of T effector cells into antigenic sites. (C) Inhibition of mTOR via rapamycin or genetic deletion reduces protein synthesis, and promotes T cell differentiation towards anergic and Treg subsets. Rapamycin-mediated inhibition of mTOR causes T effector cells to re-express CD62L and CCR7 and home to SLOs.