Abstract
Understanding the structural development of embryonic bone in a three dimensional framework is fundamental to developing new strategies for the recapitulation of bone tissue in latter life. We present an innovative combined approach of an organotypic embryonic femur culture model, microcomputed tomography (μCT) and immunohistochemistry to examine the development and modulation of the three dimensional structures of the developing embryonic femur. Isolated embryonic chick femurs were organotypic (air/liquid interface) cultured for 10 days in either basal, chondrogenic, or osteogenic supplemented culture conditions. The growth development and modulating effects of basal, chondrogenic, or osteogenic culture media of the embryonic chick femurs was investigated using μCT, immunohistochemistry, and histology. The growth and development of noncultured embryonic chick femur stages E10, E11, E12, E13, E15, and E17 were very closely correlated with increased morphometric indices of bone formation as determined by μCT. After 10 days in the organotpyic culture set up, the early aged femurs (E10 and E11) demonstrated a dramatic response to the chondrogenic or osteogenic culture conditions compared to the basal cultured femurs as determined by a change in μCT morphometric indices and modified expression of chondrogenic and osteogenic markers. Although the later aged femurs (E12 and E13) increased in size and structure after 10 days organotpypic culture, the effects of the osteogenic and chondrogenic organotypic cultures on these femurs were not significantly altered compared to basal conditions. We have demonstrated that the embryonic chick femur organotpyic culture model combined with the μCT and immunohistochemical analysis can provide an integral methodology for investigating the modulation of bone development in an ex vivo culture setting. Hence, these interdisciplinary techniques of μCT and whole organ bone cultures will enable us to delineate some of the temporal, structural developmental paradigms and modulation of bone tissue formation to underpin innovative skeletal regenerative technology for clinical therapeutic strategies in musculoskeletal trauma and diseases.
Introduction
Significant challenges in tissue engineering still exists for the generation of complex tissues/organs that can only be informed by a comprehensive understanding of the developing tissue environment. Ex vivo organ cultures have historically provided valuable insights into the differentiation and developmental growth patterns of collective cell populations concomitant in their customary complex three-dimensional tissue environment. Information between the aspects of in vitro skeletal cell/matrix modulation and in vivo bone development/regeneration has previously been achieved by studying skeletal ex vivo organ cultures and embryonic limb rudiment cultures.1–4
Organ culture systems allow the relationship of skeletal cells within their extracellular matrix to be maintained while the external environment can be manipulated by a variety of external factors. This allows gene and protein expression, structural growth patterning and remodeling to be systematically evaluated during the embryonic bone growth and mineralization stages.5–8 An example of such a model is the embryonic chick bone organ culture system, which has the capacity to be a rapid throughput model for the investigation of bone development and regeneration.9,10 Bone organ culturing systems such as the development of the organotypic (air/liquid interface) models have improved our understanding of the development of tissue and the modulating effects of external stimuli on them. This model that has the tissue residing on a membrane that is in contact with a minimal amount of culture medium, allows the analysis of the interaction of many cell phenotypes in the tissue in a three-dimensional setting without the loss of the tissue integrity. However, despite advances in cellular, molecular, and immunohistochemical techniques the data typically obtained from organotypic models remains, to date, semiquantitative with limited information on the formation and bone patterning sequences within a three-dimensional structure.
For over four decades, following its clinical inception in the 1970s11 microcomputed tomography (μCT) systems have been an influential tool in providing high-resolution quantitative information in the spatial patterning and the integral three-dimensional skeletal density, morphology, and trabecular architecture occurring in embryonic bone development, postnatal growth, epigenetic influences, disease, and skeletal regenerative strategies.11–13 Although μCT provides superior analysis of three-dimensional skeletal morphology, μCT is not without its limitations with regard to provision of data in understanding fully the rudiments of bone development.
Here, we demonstrate that by using chick embryonic femur organotypic cultures we can investigate the development of bone with great precision in a three-dimensional setting and explore the effects of various supplemented culture conditions on the developmental architecture of the bone. These combined methods can be used for rapid, high-throughput, nondestructive screening of ex vivo bone growth and development, and importantly enhance our understanding of skeletal developmental biology providing critical insights into developing new paradigms for tissue engineering approaches to skeletal regeneration and repair.
Materials and Methods
Materials
Six-well tissue culture plates were obtained from Greiner BioOne. Millicell culture inserts (0.4 μm pore size; Cat No. PICM03050) were purchased from Millipore (30 mm diameter). Cell Tracker Green™ (CMFDA) and Ethidium Homodimer-1 were purchased from Invitrogen, Scotland. Phosphate Buffered Saline (PBS) was purchased from Lonza Biologics. Dexamethasone, ascorbic acid 2-phosphate, and all other tissue culture media were obtained from Sigma-Aldrich, UK unless stated. LF68 type I collagen antibody was a kind gift from Larry Fisher at the NIH, Bethesda, MD. Type II collagen antibody (rabbit polyclonal) was purchased from Calbiochem, and the STRO-1 antibody undiluted culture supernatant was obtained from the STRO-1 hybridoma provided by Dr J. Beresford, University of Bath.
Methods
Differential staining of cartilage and bone in whole chick embryo by Alcian blue and Alizarin red S
Chick embryos (Callus domesticus) were isolated at days 9–15 of gestation and double stained for Alcian blue and Alizarin red based on the procedure previously adopted and described14,15 to assess the rate of skeletal development in the chick over the gestation period. This gave us an indication of the optimum ages for the manipulation of the embryonic femur in the organotypic culture set up. Briefly, chick embryos were culled and fixed in 4% paraformaldehyde (PFA), then placed in 100% ethanol for 4 days, and then placed in 100% acetone for a further 4 days to remove the fat. After rinsing in distilled water the embryos were stained using Inouye solution (0.003% Alcian blue, 0.001% Alizarin red, 60% EtOH, and 5% acetic acid) for 3 days. The tissue was then cleared using 1% KOH (w/v in dH2O) until the skeleton was clearly visible. The embryos were further cleared using 1% KOH+20% Glycerin. The stained skeletons were then stored in 80% glycerine and photographed using a Power shot G10 Camera (Canon).
Isolation of embryonic chick femurs
Femora were dissected from 9, 10, 11, 12, 13, 15, or 17-day-old chick embryos (Callus domesticus), where the soft tissue such as adherent muscles and ligaments were carefully removed while preserving the periosteum. These noncultured femurs were immediately fixed in 85% ethanol or 4% paraformaldehyde where they were scanned using μCT.
Organotypic cultures of embryonic chick femurs
The dissected femurs for organotypic cultures were washed in 1× PBS and placed in the organotypic set up (Fig. 1A–C). The bones were transferred to six-well plates and positioned resting on 0.40 μm filter well inserts at the interface between the air and the basal culture media (1 mL of basal tissue culture media (TCM) consisting of α-minimum essential medium (α-MEM), penicillin (100 U/mL), streptomycin (100 μg/mL), and ascorbic acid 2-phosphate (100 μM)). The cultures were maintained in the basal media for 24 h at 37°C in humidified air with 5% CO2. The organotypic cultures were then incubated in different culture conditions–basal TCM (1 mL), chondrogenic TCM (1 mL; consisting of basal TCM+ transforming growth factor-β3 (10 ng/mL), insulin, transferrin, selenium, and dexamethasone (10 nM)); osteogenic TCM (1 mL; consisting of basal TCM+ (10 nM) dexamethasone) providing an air/liquid interface with the femur. A final submerged femur basal TCM group was added where the femurs were exposed to 2 mLs of basal culture media instead of 1 mL. Culture media was changed daily for the duration of the experiment (10 days) (n=4 femurs per group).
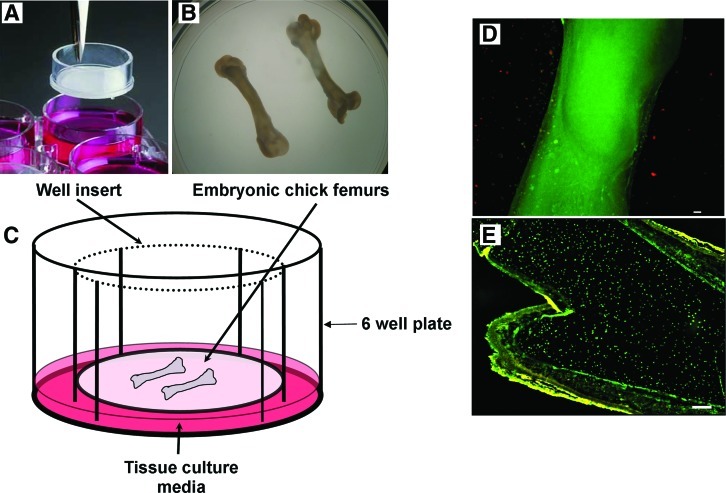
FIG. 1.
The embryonic chick femur organotypic culture set up. (A) 0.44 μm filter membrane inserts positioned in six-well plates. (B) Embryonic chick femurs were isolated and placed on the membrane and (C) culture media was then added to the six-well plates to create an air/liquid interface. Cell viability and histological analysis of organotypic cultured femurs. Cell viability (cell tracker green (CMFDA)/ethidium homodimer-1) of embryonic chick femurs (E13) organotypic cultured in (D) osteogenic conditions for 10 days and examined by fluorescent microscopy showing viable cells (green) and negligible associated necrosis (red). (E) A representative tissue section from (E13) embryonic chick femur cultured in osteogenic conditions displaying viable cells throughout the organotypic cultured femur (scale bar=100 μm). Color images available online at www.liebertonline.com/tec
At the end of the experiments, embryonic femurs were incubated with Cell Tracker Green™ (CMFDA) and Ethidium Homodimer-1 (CTG/EH-1) to assess the viability of the femora cultured in the organotypic set up. Subsequently, all noncultured femurs (Day 9, 10, 11, 12, 13, 15, and 17) and organotypic cultured femurs were washed in 1× PBS and either fixed in 85% ethanol or 4% paraformaldehyde. The femur samples were then imaged radiographically using a Faxitron® Specimen Radiography System (MX-20) (Qados Ltd.) and the lengths measured. The noncultured and organotypic cultured femurs were then scanned using μCT and then processed for histology and analyzed for proteoglycan, collagen (type I an II) levels, mineralization, and STRO-1 expression.
Microcomputed tomography
Quantitative 3D analysis of noncultured embryonic chick femurs (Day 10, 11, 12, 13, 15, and 17) and organotypic embryonic chick femurs (Day 10, 11, 12, and 13) cultured for 10 days was performed using an Xtek BenchTop 160Xi CT scanning system for microcomputed tomography (X-TEK Systems Ltd.) equipped with a Hamamatsu C7943 X-ray flat panel sensor (Hamamatsu Photonics). The femur samples were centered in the middle of the μCT X-TEK machine, focused, calibrated, and adjusted to prevent X-ray saturation of the sample. Femur samples were scanned using settings 60 kV, 150 μA with a molybdenum target with an exposure time of 1067m/s, 2× digital gain, number of angular positions at 701, and scans performed with minimized ring artifacts. Raw data was collected and reconstructed using CTPro (X-TEK Systems Ltd.) with a mean 11 μm voxel resolution. The reconstructed femurs were selected for quantification of bone. The reconstructed images were visualized and analyzed using Volume Graphics Studio Max 1.2.1 software package (Volume Graphics, GmbH). After simple bone segmentation thresholds were used to remove the soft tissue; bone 3D images were created and saved as TIFF and JPEG interchangeable files and analyzed for macro- and microscopic structural properties. Bone Volume (BV), Bone Surface/Bone Volume (BS/BV), Bone Volume/Total Volume (BV/TV), Trabecular Thickness (Tb.Th), Trabecular Number (Tb.No), and Trabecular Spacing (Tb.Sp) were then calculated using the computational algorithms developed by X-Tek.
Histological and immunohistochemical analysis of the organotypic femur cultures
Following μCT analysis, embryonic chick femur samples were dehydrated in a graded series of alcohols and embedded in low-melting point paraffin using an automated Shandon Citadel 2000. Six micrometers tissue sections were cut from across the femur sample and stained for the nuclear counter-stain Weigert's hematoxylin, followed by staining with 0.5% Alcian blue 8GX for proteoglycan-rich cartilage matrix and 1% Sirius red F3B for collagenous matrix. Sections were also stained with von Kossa for assessing bone mineralization. Additionally, femur samples were analyzed for type I collagen, type II collagen, and STRO-1 expression. In brief, after quenching endogenous peroxidase activity with 3% H2O2 and blocking with 1% bovine serum albumin (BSA) in 1× PBS, sections were incubated overnight at 4°C with primary antibodies diluted appropriately in 1% BSA in PBS: either LF68 type I collagen (1:1000), type II collagen (1:500), or STRO-1. An additional hyaluronidase incubation step was included prior to incubation with type II collagen antibody. Additionally, we investigated the level of expression of CD31 (GeneTex) in noncultured and organotypic cultured (E11) femurs (basal culture conditions) to determine whether an early form of a vasculature were present in these aged femurs.
Following primary antibody incubations, sections were incubated with biotin-conjugated secondary antibodies, diluted appropriately in 1% BSA in PBS: anti-rabbit IgG (DAKO A/S; 1:100) for type I, type II collagen, and CD31, or anti-mouse IgM for STRO-1. Visualization of the immune complex involved the avidin-biotin method linked to peroxidase and 3-amino-9-ethylcarbazole, resulting in a reddish brown reaction product. Sections were counterstained for light green and Alcian blue and mounted in aqueous CC/Mount (Sigma). No staining was observed in any control sections in which the primary antibody was omitted. IgM isotype controls were run alongside the STRO-1 antibody-incubated tissue sections.
Whole slide images of the histological and immunohistochemical stained sections were analyzed using an Olympus BX-51/22 dotSlide microscope and images created using OlyVIA 2.1 software (Olympus Soft Imaging Solutions, GmBH), and by a Carl Zeiss Axiovert 200 microscope with Carl Zeiss Axiovision 3.1 software package used to capture images.
Time lapse movie of organotypic culture of E11 embryonic chick femurs
E11 femurs were placed in the organotypic culture set up and placed on a humidified sealed stage (5%CO2 balanced air) Carl Zeiss Axiovert 200 microscope and imaged over a 24 h incubation period. A Carl Zeiss Axiovision 3.1 software package was used to capture the movie of the developing organotypic femur.
Statistical analysis
All measurements were calculated as mean±standard deviation. Statistical analysis was performed using GraphPad Prism 3.02 software. Differences among groups were determined by one-way analysis of variance with a post-hoc Dunnett's test and statistical differences were considered to be significant if p<0.05.
Results
Differential staining of cartilage and bone in the developing chick embryo by Alcian blue and Alizarin red S
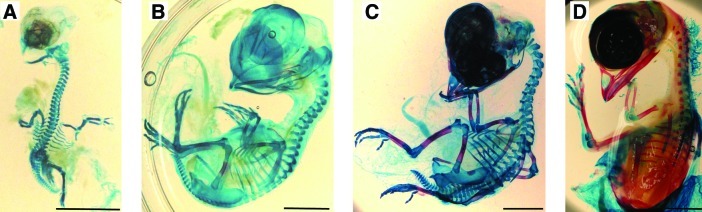
Microscopic evaluation of the Inouye skeletal-stained embryonic chick specimens demonstrated rapid ossification of bone and cartilage from gestation age Day 9–Day 15 (Fig 2A–D). Day 11 chick embryos were evaluated as the optimal point where skeletal differentiation and bone mineralization were just starting, as indicated by the stained femur (Fig 2B). This was later confirmed by μCT (Fig 3A and C).
FIG. 2.
Alizarin red (bone) and Alcian blue (cartilage) skeletal staining of representative embryonic chicks at different stages of development (A) E9, (B) E11, (C) E13, and (D) E15. (Scale bar=10 mm). Color images available online at www.liebertonline.com/tec
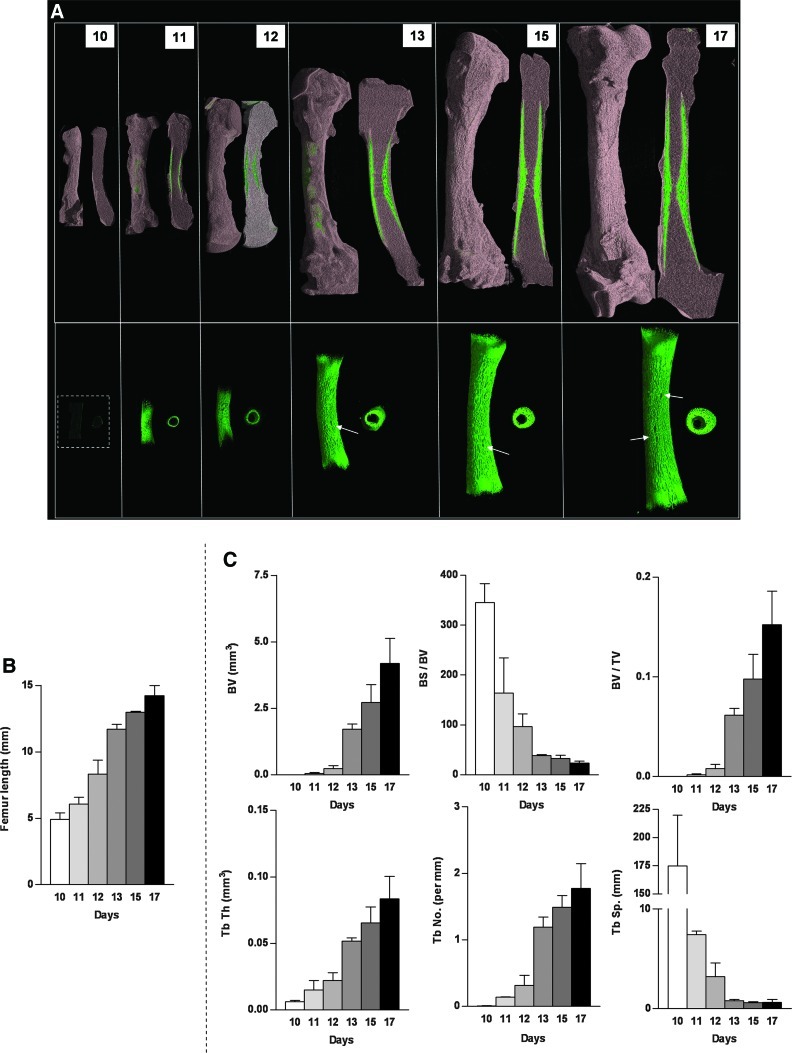
FIG. 3.
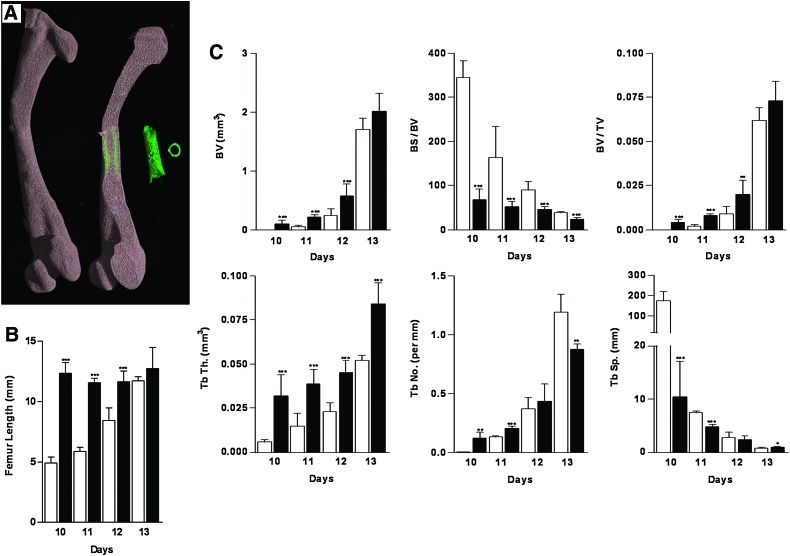
Microcomputed tomography (μCT) images and analysis of the developing embryonic chick femur. (A) μCT images (whole femur tissue; saggital sections; segmented mineralized bone (green); and cross-sectional sections of the central diaphysis region) of the embryonic chick femurs depicting the bone growth from E10–E17. (B) Analysis of the length (mm) of noncultured femurs from embryonic chicks E10–E17. (C) μCT morphometric indices of the noncultured E10–E17 embryonic chick femurs. Values are mean±SD (n=6 femurs per group). BS, bone surface; Tb Th, trabecular thickness; Tb Sp, trabecular spacing; Tb No, trabecular number; BV, bone volume; TV, total volume. Color images available online at www.liebertonline.com/tec
μCT structural analysis of developing embryonic bone
High-definition μCT analysis of the structural development of noncultured embryonic chick femurs demonstrated and highlighted the rapid increase in the bone development between stages E10, E11, E12, E13, E15, and E17 (Fig. 3A). The detail in the architecture of the bone demonstrated distinct pores in the diaphyseal regions (arrows) of the developing E13, E15, and E17 femurs (Fig. 3A). The μCT images depicting the growth of the embryonic femur correlated well with increases in femur length (Fig. 3B) and increases in 3-D μCT morphometric indices for trabecular bone architecture for BV, BV/TV, Tb.Th, Tb.No, and reduced BS/BV and Tb.Sp (Fig. 3C).
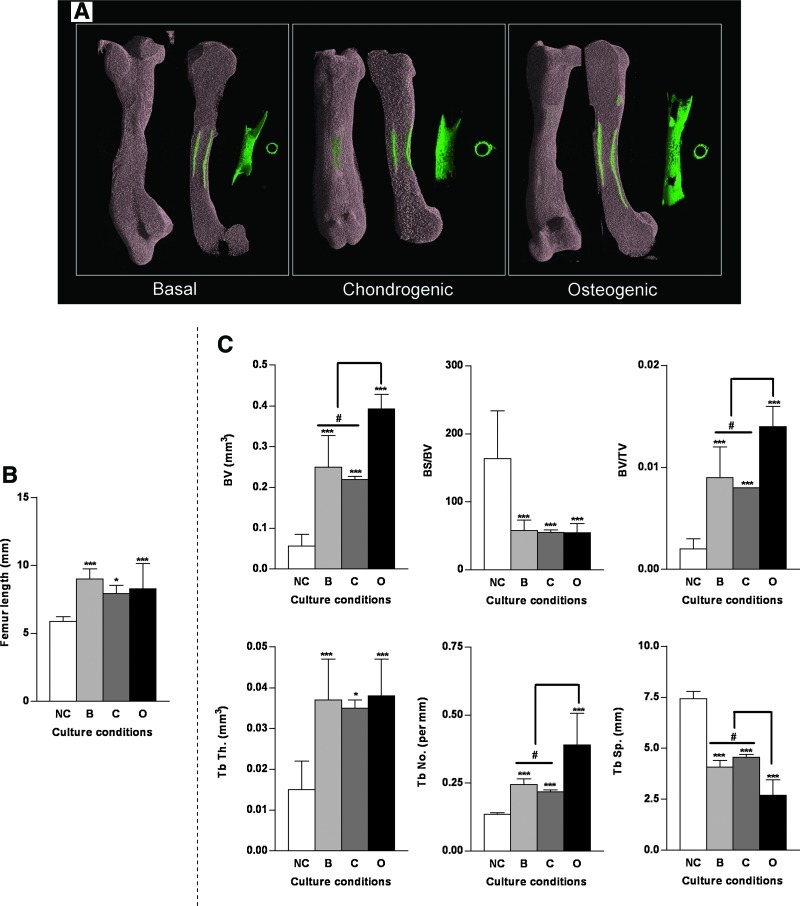
Organotypic cultures of embryonic bone (basal, chondrogenic, and osteogenic conditions)
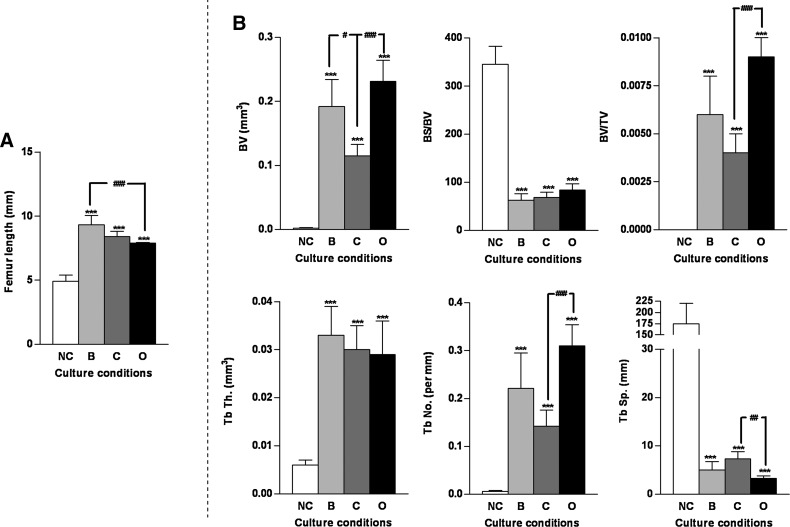
Organotypic cultures (Fig. 4A) undertaken in the presence and absence of appropriate chondrogenic and osteogenic chemical cues (basal, chondrogenic, and osteogenic media) was used to examine the structural morphology and development of the embryonic bone. Significant differences in the structural architecture of the embryonic bones (E11) were observed between basal, chondrogenic, and osteogenic organotypic cultures over a 10 day culture period. Embryonic chick femurs cultured in chondrogenic conditions were observed to be shorter in stature with a thicker diaphysis and an enlarged epiphyseal region compared with basal cultured femurs (Fig. 4A). In the osteogenic culture conditions embryonic chick femurs were comparable to the basal cultured femurs, however, the levels of bone mineralization were increased compared with the embryonic bones cultured in basal and chondrogenic conditions (Fig. 4A). Significant increases in femur length were observed in the three organotypic cultured groups over 10 days compared with the control E11 noncultured femurs (Fig. 4B). μCT analysis confirmed that the E11 embryonic femurs cultured in osteogenic conditions displayed a significantly elevated structural content as indicated by increased BV, BV/TV, Tb.No, and reduced Tb.Sp (Fig. 4C) compared with basal and chondrogenic cultured embryonic chick femurs. Interestingly, the mean Tb.Th in each one of the embryonic femurs cultured in the different conditions was surprisingly similar (Fig. 4C).
FIG. 4.
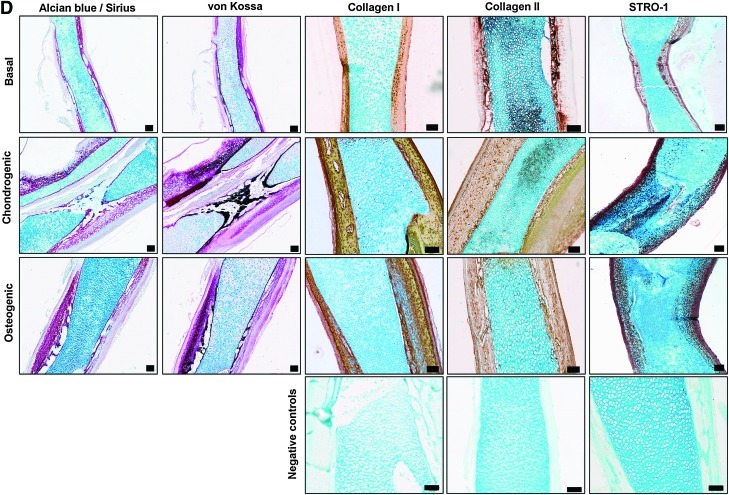
μCT and histological analysis of organotypic cultured embryonic femurs in basal, chondrogenic, and osteogenic conditions. (A) μCT images (whole femur tissue; saggital sections; segmented mineralized bone (green); and cross-sectional sections of the central diaphysis region) of E11 embryonic chick femurs organotypic cultured in basal, chondrogenic, and osteogenic media for 10 days. (B) Femur lengths *p<0.05; ***p<0.001 increase in femur length compared with noncultured femurs. (C) μCT morphometric indices of the structure of the organotypic cultured embryonic chick femurs E11 (NC=noncultured, B=basal, C=chondrogenic, O=osteogenic). Values are mean±SD (n=4 femurs per group) *p<0.05; ***p<0.001 increase or decrease in μCT bone morphometric indices of basal, chondrogenic, and osteogenic cultured femurs compared to noncultured (NC) femurs. #p<0.05 increase/decrease in μCT bone morphometric indices of osteogenic cultured E11 femurs compared to basal and chondrogenic cultured femurs. (D) Histological analysis of the embryonic femurs cultured in the three conditions for Alcian blue/Sirius red, von Kossa, expression of collagen type I and II and STRO-1+ (scale bar=100 μm). Representative immunohistochemistry negative control (primary antibody omission) staining of type I collagen, type II collagen, and STRO-1 of embryonic chick femurs (E11) after 10 days in the organotypic cultures. Color images available online at www.liebertonline.com/tec
The bone structural differences determined by μCT analysis correlated with the histological evaluation of the corresponding femurs. The bone collar of the diaphyseal regions of the femurs were enlarged in both the osteogenic and chondrogenic cultured conditions compared with basal conditions, with enhanced mineralization (von Kossa) occurring in the osteogenic cultured femurs (Fig. 4D). Elevated levels of type I collagen expression were associated with the osteogenic cultured femurs. Type II collagen expression was associated more in the basal and chondrogenic cultured femurs compared with the osteogenic cultured femurs with a high degree of type II collagen expression in the central femur/chondrocyte region and the periosteal bone collar region. Further, expression of the skeletal stem cell enrichment marker STRO-1 was elevated in correlation with the thickening of the periosteal bone collar in the osteogenic and chondrogenic cultured femurs (Fig. 4D).
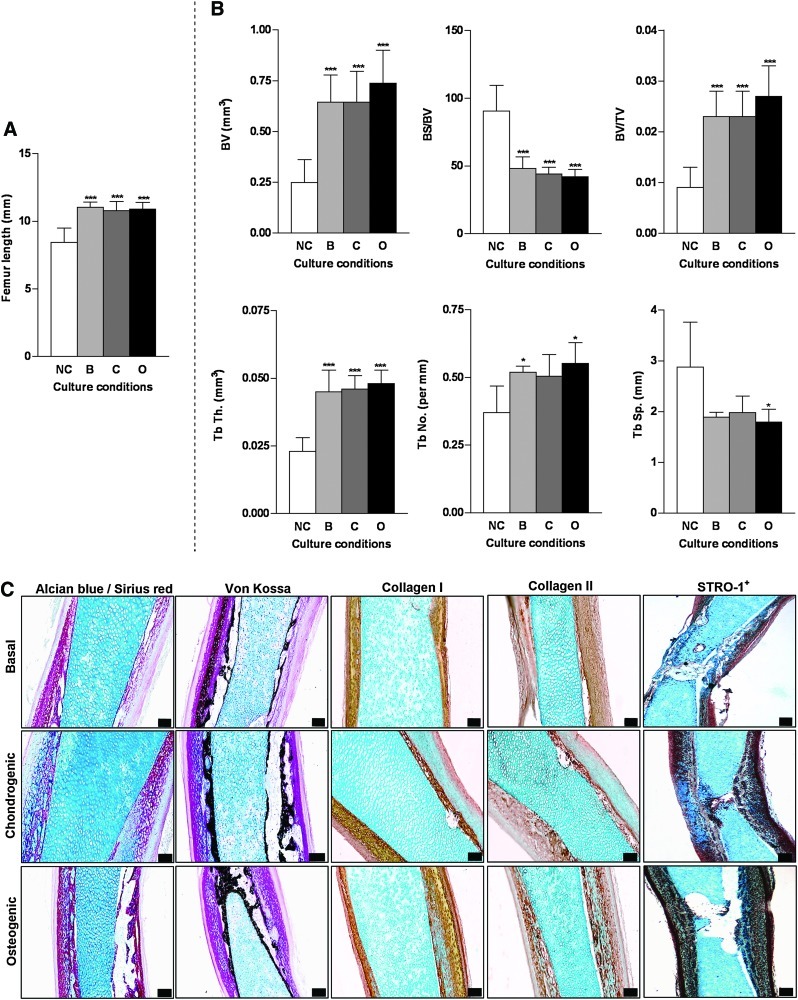
μCT morphometric analysis of the organotypic cultures at stages E10, E12, and E13 allowed delineation of the different responses present when placed in the basal, chondrogenic, and osteogenic culturing conditions. Organotypic osteogenic cultures of E10 embryonic femurs demonstrated a significant decrease in size of the femurs compared with femurs cultured in basal conditions (Fig. 5A). However, comparable bone structural development as observed in the organotypic cultures of E11 embryonic femurs were demonstrated in the E10 organotypic cultures (Fig. 5B), where the osteogenic culture groups demonstrated significant differences in its structural content compared to the chondrogenic culture femur groups. E10 femurs were on the very limits of the detectable range for bone mineralization with E9 and E10 embryonic femurs representing a predominantly cartilage anlagen, but with the potential to develop into a mineralized tissue in the three culturing conditions. E12 embryonic femurs demonstrated significant increases in bone growth (Fig. 6A) and changes in structural bone morphometric indices over the 10 day culture period in the three culture groups compared with the uncultured E12 embryonic femurs. However, no significant differences in the structural bone morphometric indices were observed between the three culture groups (Fig. 6B). Very little differences were observed in the histological analysis of the embryonic chick femurs in the various organotypic culture conditions. The only noted differences were an increase in STRO-1 expression in the bone collar and periosteum of the chondrogenic and osteogenic cultured femurs compared with the basal cultured femurs (Fig. 6C).
FIG. 5.
μCT analysis of organotypic cultured embryonic femurs (E10) in basal, chondrogenic, and osteogenic conditions. (A) Femur lengths; ***p<0.001 increase in femur length compared with noncultured femurs; ###p<0.001 increase in femur length basal compared with osteogenic femur culture conditions. (B) μCT morphometric indices of the structure of E10 embryonic chick femurs organotypic cultured in basal, chondrogenic, and osteogenic media for 10 days. Values are mean±SD (n=4 femurs per group). μCT morphometric indices of the structure of the organotypic cultured embryonic chick femurs E10 (NC=noncultured, B=basal, C=chondrogenic, O=osteogenic). Values are mean±SD (n=4 femurs per group). ***p<0.001 increase or decrease in μCT bone morphometric indices of basal, chondrogenic and osteogenic cultured femurs compared to noncultured (NC) femurs. #p<0.05 decrease in BV between basal and chondrogenic cultured femurs. ##p<0.01; ###p<0.001 increase/decrease in μCT bone morphometric indices of osteogenic cultured E10 femurs compared to basal and chondrogenic cultured femurs.
FIG. 6.
μCT and histological analysis of organotypic cultured embryonic femurs (E12) in basal, chondrogenic, and osteogenic conditions. (A) Femur lengths; ***p<0.001 increase in femur length compared with noncultured femurs. (B) μCT morphometric indices of the structure of E12 embryonic chick femurs organotypic cultured in basal, chondrogenic, and osteogenic media for 10 days. Values are mean±SD (n=4 femurs per group). μCT morphometric indices of the structure of the organotypic cultured embryonic chick femurs E12 (NC=noncultured, B=basal, C=chondrogenic, O=osteogenic). Values are mean±SD (n=4 femurs per group). *p<0.05 ***p<0.001 increase or decrease in μCT bone morphometric indices of basal, chondrogenic, and osteogenic cultured femurs compared to noncultured (NC) femurs. (C) Histological analysis of the E12 embryonic femurs cultured in the three conditions for Alcian blue/Sirius red, von Kossa, expression of collagen type I and II and STRO-1+ (scale bar=100 μm). Color images available online at www.liebertonline.com/tec
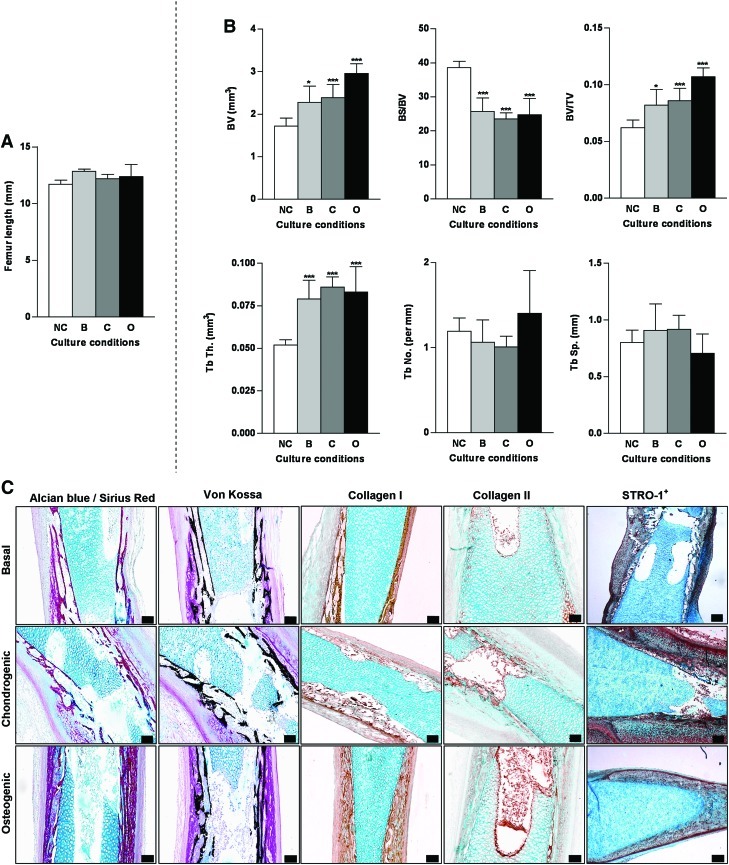
Conversely, E13 embryonic femurs displayed no significant increase in growth in any of the 10 day cultured femur groups compared to noncultured embryonic femurs (Fig. 7A), although the degree of some of the bone morphometric indices determined by μCT, was significantly increased/decreased in all the cultured femur groups compared with the noncultured femurs. Compared with other aged femurs (E10-E12) the trabecular structure was significantly thicker but the number and spacing remained the same as the noncultured embryonic femur (Fig. 7B). In the comparative histological analysis, only the levels of STRO-1 expression in the chondrogenic cultured femur group were observed to increase compared with other culture groups (Fig. 7C).
FIG. 7.
μCT and histological analysis of organotypic cultured embryonic femurs (E13) in basal, chondrogenic, and osteogenic conditions. (A) Femur lengths. (B) μCT morphometric indices of the structure of E13 embryonic chick femurs organotypic cultured in basal, chondrogenic, and osteogenic media for 10 days. Values are mean±SD (n=4 femurs per group). (NC=noncultured, B=basal, C=chondrogenic, O=osteogenic). *p<0.05 ***p<0.001 increase or decrease in μCT bone morphometric indices of basal, chondrogenic, and osteogenic cultured femurs compared to noncultured (NC) femurs. (C) Histological analysis of the E13 embryonic femurs cultured in the three conditions for Alcian blue/Sirius red, von Kossa, expression of collagen type I and II and STRO-1+ (scale bar=100 μm). Color images available online at www.liebertonline.com/tec
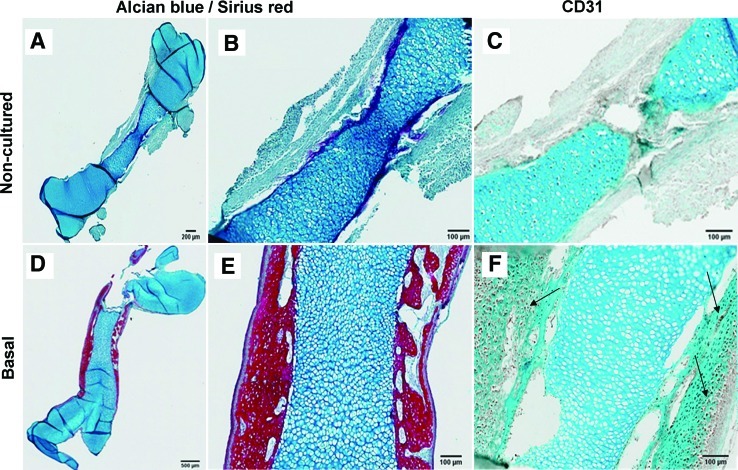
In contrast, embryonic femurs (E10-12) that were not cultured in an air/liquid interface but were submerged in basal TCM for the 10 day culture period were observed to be very elongated with a distended, enlarged epiphyseal region and a diminutive, reduced diaphyseal bone collar (Fig. 8A). All cultured femurs E10-E12 significantly increased in length over the 10 day culture period compared with the noncultured femurs. However, this was not the case for the more developed E13 embryonic chick femurs (Fig. 8B). Similarly, submerged E10-E12 cultured femurs demonstrated significant modulation of the bone morphometric indices as determined by μCT (Fig. 8B). The one exception was the Tb.No and Tb.Sp readings of submerged E12 embryonic chick femurs where no significant increases compared with the noncultured femurs were observed. Again, for the more developed embryonic femur (E13) there was a different response when cultured for 10 days. The femur length, BV, and BV/TV levels did not increase compared to noncultured femurs but the trabecular thickness was significantly higher than noncultured (E13) femurs. Interestingly, in contrast to the E10-E12 submerged cultured bones there was a decrease in the trabecular number and an increase in trabecular spacing of the E13 submerged cultured bones when compared with the noncultured bones (Fig. 8C). There was negligible expression of the vascular marker CD31 in the noncultured E11 femurs. However, in the organotypic femurs (E11) cultured under basal conditions for 10 days, we found an enhanced expression of CD31 in the cells of the cortical/periosteal region of these femurs (Fig. 9).
FIG. 8.
μCT analysis of embryonic chick femurs (E10-E13) cultured in submerged basal media for 10 days. (A) μCT images (whole femur tissue; saggital sections; segmented mineralized bone (green); and cross-sectional sections of the central diaphysis region) of embryonic chick femurs (E11). (B) Analysis of embryonic chick femur growth (E10-E13) cultured in submerged media conditions (▪) compared to noncultured femurs (□). Values are mean±SD (n=4 femurs per group) ***p<0.001 increase in femur length compared with noncultured femurs of the equivalent age. (C) μCT morphometric indices of the structure of the embryonic chick femur (E10-E13) cultured in submerged media conditions (▪) compared to noncultured femurs (□). Values are mean±SD (n=4 femurs per group). *p<0.05; **p<0.01, ***p<0.001 increase/decrease in μCT bone morphometric indices; submerged cultured embryonic chick femurs compared to noncultured femurs. Color images available online at www.liebertonline.com/tec
FIG. 9.
Histological analysis of E11 embryonic femurs cultured in basal conditions for Alcian blue/Sirius red and CD31 expression. Alcian blue and Sirius red staining for (A, B) noncultured E11 embryonic femurs and (D, E) E11 organotypic cultured femurs (10 days). CD31 expression (arrows) in (C) noncultured E11 embryonic femurs and (F) E11 organotypic cultured femurs (10 days). Color images available online at www.liebertonline.com/tec
Discussion
Previously, the complex orchestrated events that determine patterns of skeletal development have been largely established through cellular and molecular studies. Bone organ culture systems maintain the various cells and extracellular matrix interactions in a 3D biomimetic environment, where responses to a plethora of factors result in modulation of the bone similar to the effective response in vivo.
Using the chick model as shown by its rapid mineralization process the technique of combining an established but modified organotypic embryonic whole femur culture system with μCT analysis provides a powerful, rapid system for determining the 3D spatial patterning of fundamental bone development, correlating to the cellular and immunohistochemical data obtained. We have demonstrated that the combined techniques have provided correlative, quantitative analysis of the intricate changes associated with the addition of external factors to the organotypic embryonic femur cultures. Indeed, these femurs maintained a high degree of viability and were sufficiently nourished during the culture period allowing for possible further longitudinal studies.
Incubation of the embryonic femurs in basal, chondrogenic, or osteogenic culture conditions at the various stages of the development had marked effects on the way the bone further developed in the organotypic cultures. Osteogenic culture conditions had a profound effect on the skeletal development of early femur cultures (E10 & E11) compared with the same staged femurs cultured in basal or chondrogenic conditions. The osteogenic cultures were performed without the supplementation of β-glycerophosphate to assess the capability of the organotpyic cultured femurs to drive the mineralization process in situ without an exogenous phosphate source to stimulate the bone cells to mineralize.
To accomplish this quantification through traditional histological or imaging techniques would be an exhaustive and imprecise process. E13 cultured embryonic femurs did not significantly increase in length after 10 days in the various organotypic culture conditions compared with the E13 noncultured femurs; however, the bone parameters as assessed by μCT demonstrated an increase in all the three culture groups compared with the noncultured femurs. The thickness of the trabecular bones in the E13 cultured femurs increased compared with the noncultured bones whereas the trabecular number and spacing remained at the same level as the noncultured femurs. In contrast to the same staged femurs cultured in a submerged setting (not organotypic) a different morphology was observed. These femurs had a very small dense diaphysis, and an elongated, distended epiphyseal region. From E10-E12 the submerged cultured embryonic femurs were significantly modified in all μCT morphometric indices compared with the corresponding aged noncultured femurs. Moreover, in contrast to the E13 staged embryonic femurs that were cultured in the organotypic set up an opposite effect was observed in E13 submerged cultured embryonic femurs where the trabecular numbers were reduced and the trabecular spacing increased.
From the experiments undertaken in this study, histological and immunohistochemical analysis of these embryonic chick femurs correlated very closely to the μCT results obtained. Interestingly, throughout this study we observed varying degrees of expression of the skeletal progenitor enrichment marker STRO-1, which appeared to be modulated by the development of the femur or modulated when osteogenic or chondrogenic stimuli were applied to the organotypic femur cultures. Current studies in our lab are being undertaken to determine the role of STRO-1 in the development of the embryonic skeleton. Additionally, we found that the expression levels of the vascular marker CD31 increased in the 10 day organotypic cultured E11 compared with the negligible levels in the noncultured E11 femurs. This is very interesting as the vasculature is not present in the femur at this point in development. This model in conjunction with μCT analysis has also demonstrated that in some of the culture conditions the femurs show excavated tunnels in the diaphyseal regions of the cortical bone that has developed without the presence of proliferating endothelial cells or the nascent capillary invasion into the bone (data not shown). We are now undertaking studies to determine the level of endothelial precursors residing in the developing femur at early gestation time points.
Observations using time-lapse photography (Supplementary Video S1[available online at www.liebertonline.com/tec]) during the organotypic culture process (24 h) demonstrated the embryonic femur to expand in a twisting motion rather than a predictable straight-line extension of the bone. In fact, mechnotransduction and its effect thereon skeletal development could be easily assessed by the combination of the multidimensional systems of μCT and organotypic cultures. Indeed, bone organ cultures have been used recently to try and identify the mechanosensitivity effects on bone.16 Therefore, understanding the structural composition of skeletal patterning and how the mechanical forces of tendons and muscles exert their effects, particularly on the developing bone, will have a bearing on engineering designs for the repair and development of bone in clinical settings.
The growth and development of bone in organ cultures is still not comparable to the in vivo setting. However, an improved understanding of skeletal development, mesenchymal stem cell biology, culture techniques, and analysis techniques have demonstrated that bone organ cultures are becoming much more practical and reliable.17 The organotypic culture of embryonic bone demonstrated in our model has the capacity for high-throughput analysis of factors that will enhance and modulate the bone forming processes. In bone organ cultures the preparation of the femur will undoubtedly have some effect on the development of these femurs. However, the organotypic model appears to be robust enough for testing the effects of growth factors, hormones and other small molecules on the structural modulation of the femur. This model is based on the femur, which is predominantly driven by endochondral bone formation compared to other bone organ cultures that are based on the calvarial bone that develops through intramembranous formation.
Previous bone organ culture models have primarily been used to investigate bone resorption rather than bone formation. This is because the organ culture models used in these investigations tend to use later developed femurs9 where they have a natural tendency for activation of resorption, which has been ideal to test the efficacy of anti-resorptive agents on bone formation.18–21 We found that in our organotypic model, the earlier staged femurs respond better to osteogenic stimuli compared with the later aged femurs, probably due to the early gestation femurs being programed to drive more of a bone formation process rather than a bone remodeling process.
In our sets of studies, the organotypic femur cultures rest on hydrophilic polytetrafluoroethylene (PTFE) membranes (0.4 μm pore size) to maintain a static, close contact air/interface culture. This is an improvement on the classical metal grids16,22,23 and tissue culture plastic20 or suspension organ culture technique,24,25 where the contact of TCM with the bone tissue on the metal grids is reduced; therefore, increasing the chance of tissue necrosis or in the case of suspension organ cultures the femur is subjected to mechanical forces that may be exerted on the rotating femur. Additionally, our model allows for the μCT scanning of the fragile femur in situ in the organotypic plate. In comparison to other models the inexpensive chick femur model allows for the high-throughput analysis of bone forming or bone destructive agents without having to undertake large in vivo studies.
This model can be utilized to investigate the repair mechanisms of critical sized defects (data not shown) allowing us to determine the efficacy of repair strategies of these bones at the early embryonic stages without the presence of mechanical stimulation or a vasculature. In this study we have only employed μCT and imunohistochemistry as analyzing tools for bone development, however, this model is robust and reliable enough to use other techniques such as molecular biology, in situ hybridization, and electrophysiology that have been successfully used in other studies to investigate the physiological and pathophysiological processes in organotypic tissue slices.26
The day 10, 11 femurs appear to be the most responsive to external stimuli making it very unique. However, the model does not account for the role of vascularization and mechanical forces that would normally occur in the developing femur in vivo. Developing in vitro bioreactors that can mimic the forces that the femur would endure during development in vivo would enhance the organotypic models investigated in this study. Additionally, the chick chorioallantoic membrane model might provide the necessary angiogenic component (essential for skeletal development) to investigate the structural development in the embryonic chick femurs.
The use of high-resolution μCT analysis to evaluate cortical and trabecular bone structure has transformed our knowledge in determining the structural component and development of the skeleton. This study has engaged the use of μCT analysis and a novel chick embryonic femur organotypic culture method as a rapid multidimensional model system to enhance our knowledge based on current models to provide critical information in the 3D spatial patterning of skeletogenesis, and how varying stimuli or conditions can modulate this growth.
The technique can be extrapolated to mammalian skeletal models, particularly as the chick long bone development slightly differs from mammalian osteogenesis.27 Moreover, the techniques used in these experiments allow for the testing and analysis of the modulated embryonic femurs compared to normal femurs in a way that has not been possible till now. This multisystem set up could be in the future applied to epigenetic studies on bone development28,29 and has the potential to delineate the repair mechanisms of bone defects and bone tissue engineering regenerative models.30,31
We believe that these combined techniques can provide an integral methodology for refining factors for further in vivo analysis and hence reduce the number of test substances that researchers usually require to evaluate in large-scale animal models.
Conclusion
Fundamental to bone regeneration and repair in a clinical setting is the creation of bone substitute products with the correct profile, dimensions, and density. Hence, understanding the way bone develops and repairs in a 3D structural format will provide critical clues for bioengineers to develop smart materials for the recapitulation of adult bone. This multidimensional technical approach has the potential to yield integral information on bone development for therapeutic strategies for skeletal regenerative technology and the possible understanding for bone malformations and disease.
Supplementary Material
Acknowledgments
We thank the late Dr. Helmtrud Roach (University of Southampton) for her helpful comments and guidance on the development of the organotypic embryonic chick femur cultures. The work carried out in this article was supported by the strategic longer and larger grant (sLOLA) from the Biotechnology and Biological Sciences Research Council, UK-grant number BB/G010579/1.
Disclosure Statement
No competing financial interests exist.
References
- 1.Fell H.B. Robison R. The growth, development and phosphatase activity of embryonic avian femora and limb-buds cultivated in vitro. Biochem J. 1929;23:767. doi: 10.1042/bj0230767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fell H.B. Mellanby E. The effect of hypervitaminosis A on embryonic limb-bones cultivated in vitro. J Physiol. 1952;116:320. doi: 10.1113/jphysiol.1952.sp004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fell H.B. Galton V.A. Pitt-Rivers R. The metabolism of some thyroid hormones by limb-bone rudiments cultivated in vitro. J Physiol. 1958;144:250. doi: 10.1113/jphysiol.1958.sp006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanske B. Razzaque M.S. Vitamin D and aging: old concepts and new insights. J Nutr Biochem. 2007;18:771. doi: 10.1016/j.jnutbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagi C. Burger E.H. Mechanical stimulation by intermittent compression stimulates sulfate incorporation and matrix mineralization in fetal mouse long-bone rudiments under serum-free conditions. Calcif Tissue Int. 1989;45:342. doi: 10.1007/BF02556004. [DOI] [PubMed] [Google Scholar]
- 6.Vortkamp A. Lee K. Lanske B. Segre G.V. Kronenberg H.M. Tabin C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 7.Meghji S. Hill P.A. Harris M. Bone organ cultures. In: Arnett T.R., editor; Henderson B., editor. Methods in Bone Biology. London: Chapman & Hall; 1998. pp. 106–126. [Google Scholar]
- 8.Hamlin N.J. Price P.A. Mineralization of decalcified bone occurs under cell culture conditions and requires bovine serum but not cells. Calcif Tissue Int. 2004;75:231. doi: 10.1007/s00223-004-0190-1. [DOI] [PubMed] [Google Scholar]
- 9.Roach H.I. Long term organ culture of embryonic chick femora: a system for investigating bone and cartilage formation at an intermediate level of organization. J Bone Miner Res. 1990;5:85. doi: 10.1002/jbmr.5650050113. [DOI] [PubMed] [Google Scholar]
- 10.Roach H.I. Induction of normal and dystrophic mineralization by glycerophosphates in long-term bone organ culture. Calcif Tissue Int. 1992;50:553. doi: 10.1007/BF00582172. [DOI] [PubMed] [Google Scholar]
- 11.Hounsfield G.N. Computerized transverse axial scanning (tomography): part 1. Description of system. Br J Radiol. 1973;46:1016. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 12.Guldberg R.E. Lin A.S.P. Coleman R. Robertson G. Duvall C. 2004 Microcomputed tomography imaging of skeletal development and growth. Birth Defects Res C Embryo Today. 2004;72:250. doi: 10.1002/bdrc.20016. [DOI] [PubMed] [Google Scholar]
- 13.Lanham S.A. Roberts C. Hollingworth T. Sreekumar R. Elahi M.M. Cagampang F.R. Hanson M.A. Oreffo R.O.C. Maternal high-fat diet: effects on offspring bone structure. Osteoporos Int. 2010;21:1703. doi: 10.1007/s00198-009-1118-4. [DOI] [PubMed] [Google Scholar]
- 14.Inouye M. Differential staining of cartilage and bone in fetal mouse skeleton by alcian blue and alizarin red S. Cong Anom. 1976;16:171. [Google Scholar]
- 15.McLeod M.J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 16.Saunders M.M. Simmerman L.A. Reed G.L. Sharkey N.A. Taylor A.F. Biomimetic bone mechanotransduction modeling in neonatal rat femur organ cultures: structural verification of proof of concept. Biomech Model Mechanobiol. 2010;9:539. doi: 10.1007/s10237-010-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitsillides A.A. Rawlinson S.C. Using cell and organ culture models to analyze responses of bone cells to mechanical stimulation. Methods Mol Biol. 2012;816:593. doi: 10.1007/978-1-61779-415-5_37. [DOI] [PubMed] [Google Scholar]
- 18.Friedman J. Raisz L.G. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science. 1965;150:1465. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- 19.Raisz L.G. Niemann I. Early effects of parathyroid hormone and thyrocalcitonin on bone in organ culture. Nature. 1967;214:486. doi: 10.1038/214486a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanczler J.M. Millar T.M. Bodamyali T. Blake D.R. Stevens C.R. Xanthine oxidase mediates cytokine-induced, but not hormone-induced bone resorption. Free Radic Res. 2003;37:179. doi: 10.1080/1071576021000040673. [DOI] [PubMed] [Google Scholar]
- 21.Klaushofer K. Hoffmann O. Stewart P.J. Czerwenka E. Koller K. Peterlik M. Stern P.H. Cyclosporine A inhibits bone resorption in cultured neonatal mouse calvaria. J Pharmacol Exp Ther. 1987;243:584. [PubMed] [Google Scholar]
- 22.Ljunggren O. Ransjö M. Lerner U.H. In vitro studies on bone resorption in neonatal mouse calvariae using a modified dissection technique giving four samples of bone from each calvaria. J Bone Miner Res. 1991;6:543. doi: 10.1002/jbmr.5650060604. [DOI] [PubMed] [Google Scholar]
- 23.Nordstrand A. Nilsson J. Tieva A. Wikström P. Lerner U.H. Widmark A. Establishment and validation of an in vitro co-culture model to study the interactions between bone and prostate cancer cells. Clin Exp Metastasis. 2009;26:945. doi: 10.1007/s10585-009-9285-4. [DOI] [PubMed] [Google Scholar]
- 24.Ueda K. Yamanaka Y. Harada D. Yamagami E. Tanaka H. Seino Y. PTH has the potential to rescue disturbed bone growth in achondroplasia. Bone. 2007;41:13. doi: 10.1016/j.bone.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Yasoda A. Ogawa Y. Suda M. Tamura N. Mori K. Sakuma Y. Chusho H. Shiota K. Tanaka K. Nakao K. Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J Biol Chem. 1998;273:11695. doi: 10.1074/jbc.273.19.11695. [DOI] [PubMed] [Google Scholar]
- 26.De Simoni A. Yu L.M. Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc. 2006;1:1439. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- 27.Nowlan N.C. Murphy P. Prendergast P.J. Mechanobiology of embryonic limb development. Ann N Y Acad Sci. 2007;1101:389. doi: 10.1196/annals.1389.003. [DOI] [PubMed] [Google Scholar]
- 28.Lanham S.A. Roberts C. Cooper C. Oreffo R.O.C. Intrauterine programming of bone. Part 1: alteration of the osteogenic environment. Osteoporos Int. 2008;19:147. doi: 10.1007/s00198-007-0443-8. [DOI] [PubMed] [Google Scholar]
- 29.Lanham S.A. Roberts C. Perry M.J. Cooper C. Oreffo R.O.C. Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos Int. 2008;19:157. doi: 10.1007/s00198-007-0448-3. [DOI] [PubMed] [Google Scholar]
- 30.Kallai I. Mizrahi O. Tawackoli W. Gazit Z. Pelled G. Gazit D. Microcomputed tomography-based structural analysis of various bone tissue regeneration models. Nat Protoc. 2011;6:105. doi: 10.1038/nprot.2010.180. [DOI] [PubMed] [Google Scholar]
- 31.Kanczler J.M. Ginty P.J. White L. Clarke N.M.P. Howdle S.M. Shakesheff K.M. Oreffo R.O.C. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.