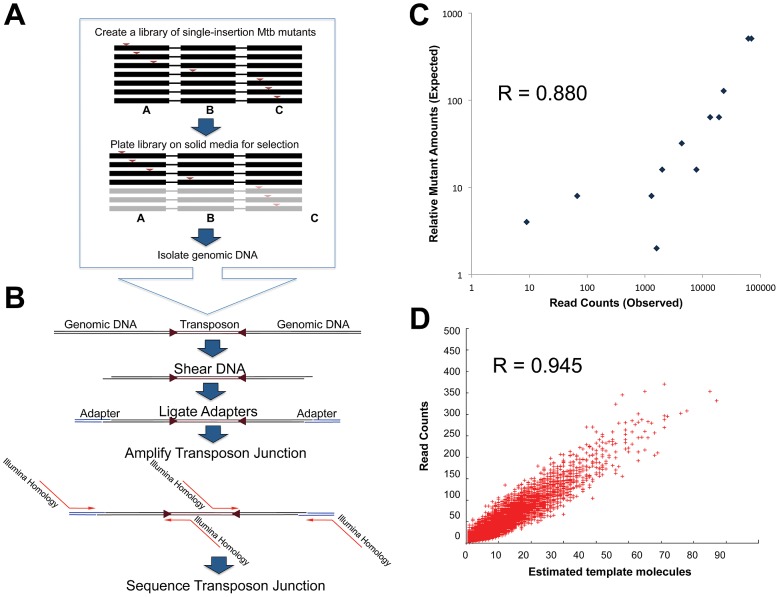
Figure 1. Transposon junction sequencing accurately reflects true library content.
A. A Mtb mutant library is created by phage-delivery of transposons, disrupting each genome with a single insertion. Shown is a schematic of 6 mutant chromosomes spanning three genes (A–C), with transposons—red arrows—disrupting one of the three genes. After growing the library on 7H10 media, we pooled surviving mutants. In this schematic, gene C is required for optimal growth and thus mutants with transposons in gene C are lost. We isolated genomic DNA from the survivors for transposon site mapping. B. We sheared the genomic DNA by sonication, and repaired frayed ends to create blunt ends. We then used Taq polymerase to generate A-tails, allowing the ligation of T-tailed adapters. Finally, we selectively amplified transposon junctions using primers recognizing the transposon end and the adapter. Primers used for amplification contain all requisite sequences to permit direct sequencing of amplicons on an Illumina Genome Analyzer 2. C. We created a library of identified transposon insertion mutants in known relative quantities. DNA from the library was prepared for transposon junction sequencing. Insertion counts were plotted against the known relative quantity of the mutant in the library. D. To further confirm that read counts were a representation of the number of genomes in the library, we estimated the number of PCR template molecules. For each gene, we plotted the estimate of template molecule count against the read counts.