Abstract
The evolution of genetic mechanisms used to combat bacterial infections is critical for the survival of animals and plants, yet how these genes evolved to produce a robust defense system is poorly understood. Studies of the nematode Caenorhabditis elegans have uncovered a plethora of genetic regulators and effectors responsible for surviving pathogens. However, comparative studies utilizing other free-living nematodes and therefore providing an insight into the evolution of innate immunity have been lacking. Here, we take a systems biology approach and use whole genome microarrays to profile the transcriptional response of C. elegans and the necromenic nematode Pristionchus pacificus after exposure to the four different pathogens Serratia marcescens, Xenorhabdus nematophila, Staphylococcus aureus and Bacillus thuringiensis DB27. C. elegans is susceptible to all four pathogens whilst P. pacificus is only susceptible to S. marcescens and X. nematophila. We show an unexpected level of specificity in host responses to distinct pathogens within and across species, revealing an enormous complexity of effectors of innate immunity. Functional domains enriched in the transcriptomes on different pathogens are similar within a nematode species but different across them, suggesting differences in pathogen sensing and response networks. We find translation inhibition to be a potentially conserved response to gram-negative pathogens in both the nematodes. Further computational analysis indicates that both nematodes when fed on pathogens up-regulate genes known to be involved in other stress responses like heat shock, oxidative and osmotic stress, and genes regulated by DAF-16/FOXO and TGF-beta pathways. This study presents a platform for comparative systems analysis of two nematode model species, and a catalog of genes involved in the evolution of nematode immunity and identifies both pathogen specific and pan-pathogen responses. We discuss the potential effects of ecology on evolution of downstream effectors and upstream regulators on evolution of nematode innate immunity.
Introduction
The struggle against infectious diseases caused by bacteria, viruses, fungi, protozoa and metazoan parasites is an important evolutionary agent [1] leading to rapid evolutionary changes responsible for much of the complexity found in the immune system of animals [2]–[4]. However, the molecular basis for the evolution of such host-pathogen interactions is only poorly understood. Over the past ten years studies of the nematode Caenorhabditis elegans have given insight into genes essential for host immunity [5], [6] as well as identifying bacterial virulence mechanisms used by opportunistic mammalian pathogens [7], [8]. These studies (and many others) have identified various signaling pathways critical for C. elegans survival when fed an array of bacterial and fungal pathogens e.g. ERK MAP kinase, p38 MAP kinase, TGF β, programmed cell death, DAF–2/DAF–16 insulin-like receptor signaling and JNK-like MAP kinase [6], [9]–[13], as well as components such as the G-protein coupled receptor FSHR-1, bZIP transcription factor zip−2 and beta-Catenin/bar−1 which are required for an inducible pathogen response [14]–[16]. It remains to be discovered however, how important these pathways are in other nematode species and how these pathways contribute to the evolutionary trajectories of bacterial pathogenicity. A comparative approach with another nematode species would provide a first entry point to enhance our understanding of the evolutionary diversity of host (nematode) response to pathogens.
One nematode that has been used extensively for comparative studies with C. elegans is the diplogastrid species Pristionchus pacificus (see [17]). In addition to having a well characterized proteome and a fully sequenced genome [18], [19], forward and reverse genetics [20], and transgenic techniques [21], full genome microarray technology has also been developed [22] allowing genomic analysis of many different traits. P. pacificus diverged from C. elegans 250–400 million years ago [18] and during this time there have been remarkable changes in vulva development [23], [24], gonad morphogenesis [25], sex determination [26] and chemosensory behaviour [27] allowing for evolutionary and developmental comparisons with C. elegans. These two nematodes also differ in their ecological niches. C. elegans can be isolated from compost heaps, snails and rotten fruits [28], whereas P. pacificus is usually isolated from a range of scarab beetles [29]–[32]. P. pacificus, as well as other Pristionchus species live in a necromenic lifestyle, that is feeding on microorganisms growing on the carcass of beetles once they die [29].
C. elegans and P. pacificus not only live in different ecological niches, but also differ in their susceptibility to bacterial pathogens. For example, C. elegans dies when fed the human opportunistic bacteria Pseudomonas aeruginosa, Staphylococcus aureus and insecticidal Bacillus thuringiensis Cry 5B toxin whereas P. pacificus is resistant [33], [34]. More recently, a screen of about 1,400 naturally strains of Bacillus yielded three strains of Bacillus thuringiensis DB27 that are extremely toxic to C. elegans but P. pacificus remain resistant [35]. Anatomically, C. elegans and P. pacificus differ in that C. elegans contains a grinder in the posterior bulb of the pharynx that is involved in the physical lysis of bacterial food [36]. While the grinder is a typical structure of nematodes of the Rhabditidae family, no grinder exists in nematodes of the Diplogastridae family, to which P. pacificus belongs [35]–[37]. Given these strong differences in the ecology and anatomy of C. elegans and P. pacificus, these two species represent ideal candidates for studying the evolution of the genetic mechanisms of pathogen response in nematodes.
Here, we used a systems level approach by testing in parallel four different bacterial pathogens that cause distinct effects on the two nematodes. We analyzed whole genome gene expression of C. elegans and P. pacificus when fed four different pathogens (Serratia marcescens, Xenorhabdus nematophila, B. thuringiensis DB27 and S. aureus) and compared each nematode pathogen response to those fed on the control bacterium (the standard nematode lab food Escherichia coli OP50). S. marcescens is a broad host pathogen present in soil and insects that kills C. elegans [8], [38] and P. pacificus. X. nematophila is a symbiotic bacteria of the entomopathogenic nematode Steinernema carpocapsae, which utilizes the bacteria to kill insects and feed on the resulting mass of proliferating bacteria [39], and also kills both the nematodes [34]. Our B. thuringiensis DB27 strain was isolated from a dung beetle (Geotrupes spp.) and seems to be one of the most pathogenic bacteria of C. elegans reported in the literature so far, which kills C. elegans in less than sixteen hours while P. pacificus is resistant [35]. We show an unexpected level of specificity in host responses to distinct pathogens within and across species, revealing an enormous complexity of effectors of innate immunity. This study presents (i) a platform for comparative systems biology of two nematode models, (ii) a catalog of genes involved in the evolution of nematode immunity and (iii) pathogen specific and pan-pathogen responses from both C. elegans and P. pacificus.
Results and Discussion
Survival of C. elegans and P. pacificus differs when fed gram-positive pathogens
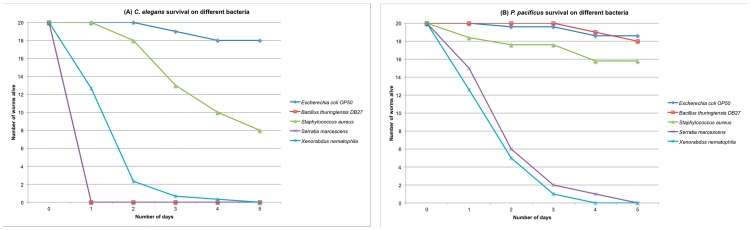
To study the evolution of the genetic mechanisms involved in nematode resistance against bacteria, we fed the four bacterial pathogens S. aureus, B. thuringiensis DB27, S. marcescens and X. nematophila to the two nematode model species C. elegans and P. pacificus and assessed their effect on survival. When C. elegans is fed monoxenic lawns of each of the four pathogens, it dies within 2–5 days (Median survival time <5 days, Figure 1A). This is in stark contrast to P. pacificus, which is more resistant to the gram-positive pathogens B. thuringiensis DB27 and S. aureus and can survive for more than 7 days (Figure 1B and Figure S1, Median survival times ∼ 8 days). However, P. pacificus, like C. elegans, is susceptible to both X. nematophila and S. marcescens and 50% of the population dies within 2–3 days exposure (Figure 1B). We would like to note here that the difference in susceptibility of P. pacificus to gram-positive bacteria tested in this study is not simply an artifact of a longer life-span, as its wild-type life-span and developmental rate is comparable to that of C. elegans ([17], [40] and our unpublished observations). Also, P. pacificus is highly susceptible (median survival ∼ 3.5 days) to a gram-positive Bacillus strain DB35 isolated from Geotrupes sp. beetles [35], indicating that it is not more resistant to gram-positive bacteria in general. We also note that P. pacificus is able to survive and reproduce on both B. thuringiensis and S. aureus indicating that its reduced susceptibility should not be due to reduced bacterial intake.
Figure 1. Differences in survival of C. elegans and P. pacificus upon exposure to different pathogenic bacteria.
The survival of C. elegans and P. pacificus is different upon exposure to different bacteria. The survival curves for (A) C. elegans and (B) P. pacificus were obtained after exposure to the gram-positive bacteria Bacillus thuringiensis DB27 and Staphylococcus aureus, and the gram-negative bacteria Serratia marcescens and Xenorhabdus nematophila. Standard lab food Escherichia coli OP50 was used as a control for both nematodes. Both nematodes show reduced survival on S. marcescens and X. nematophila. C. elegans is also susceptible to B. thuringiensis DB27 and S. aureus, while P. pacificus shows higher resistance to these two bacteria.
Significant transcriptional changes after exposure to different bacteria
To investigate the transcriptional response of the two nematodes upon exposure to these different bacterial pathogens, we identified differentially expressed genes using whole genome microarrays containing ∼43,000 probes for 20,149 C. elegans genes and ∼90,000 probes for 20,987 genes in P. pacificus respectively [22]. For each condition, total RNA was collected from four separate biological replicates of about 200 synchronized young adult worms exposed either to the pathogen or to the control E. coli (OP50) for 4 hours. The labeled cRNA produced from this total RNA was hybridized to species-specific microarrays according to manufacturer's protocols, and the raw data from scanned images was analyzed using the “limma” package in Bioconductor (see Methods for details). We observed that the exposure to pathogen resulted in a decrease of amount of total RNA produced per worm when compared to the relatively non-pathogenic E. coli strain (Figure S2). This global decrease in transcription is most likely a common feature of a core stress response, as it is also seen in case of dauer formation in both the species [22], [41] or might be an effect of the various bacteria on efficieny of RNA extraction. Nonetheless, such global transcriptional changes call for optimization of parameters used in normalization of microarray data, without which the calculated fold-changes can be erroneous [22], [42]. Our fold-change calculations take these factors into account (see Methods for details).
Although previous studies of pathogen response in C. elegans [9], [43]–[47] have looked at the transcriptome at different time-points such as 4-hours, 8-hours or 24-hours after exposure, we chose to investigate one of the earliest time-point of 4 hours because we were interested in earliest transcriptional responses manifested in response to each of these pathogens. Pathogenesis related necrosis of host-tissue at later time-points is a common effect of many pathogens and such organism-wide necrosis might dominate the expression profile at later time points [46], masking the pathogen specific signatures. Also, pathogens like Bacillus thuringiensis DB27 kill C. elegans in less than 24 hours, making analysis of late time-points unfeasible [35].
Widely different numbers of genes are found to be up- or down-regulated in C. elegans or P. pacificus upon exposure to different pathogens (Table 1A and 1B), indicating both a pathogen-specific and a nematode-specific component to these responses. In this context it is interesting to note that just a 4-hour exposure to pathogen is sufficient to cause large transcriptional changes in both the species, suggesting rapid activation of innate immune response.
Table 1. Widely different numbers of genes are differentially expressed in (A) C. elegans and (B) P. pacificus in response to the four bacteria.
| (A) C. elegans | Up | Down | TOTAL | %Up | %Down |
| B. thuringiensis | 5532 | 156 | 5688 | 97% | 3% |
| S. aureus | 181 | 68 | 249 | 73% | 27% |
| S. marcescens | 1465 | 4931 | 6396 | 23% | 77% |
| X. nematophila | 732 | 7884 | 8616 | 15% | 85% |
The genes were called differentially expressed on microarrays if the FDR corrected p-value was less than 0.05 and the absolute value of fold changes was greater than 1.42 (corresponding to log2 fold change of 0.5 where log2(1.42) = 0.5).
Changes in nematode gene expression depend on lethality and rate of killing
Based on the absolute number of differentially expressed genes under different conditions of survival, lethality and slower or faster killing rates of pathogens, some patterns can be discerned in our microarray data. First, the number of differentially expressed genes seems to be inversely correlated with the survival characteristics of the nematodes. For example, upon exposure to B. thuringiensis DB27, the pathogen most lethal to C. elegans, a remarkably large number of genes are affected in C. elegans (n = 5868, Table 1A), whereas much fewer genes (n = 217, Table 1B) are induced in P. pacificus, which is resistant to this pathogen. This can be attributed to the fact that while P. pacificus can use B. thuringiensis DB27 for food, C. elegans has to mount a robust response against a lethal pathogen.
For C. elegans, the number of differentially expressed genes is greater when exposed to faster-killing pathogens B. thuringiensis DB27, X. nematophila and S. marcescens as compared to that on S. aureus, where worms survive longer (Table 1A). Similarly, in P. pacificus, greater number of genes is differentially expressed on more lethal pathogens X. nematophila and S. marcescens (Table 1B) as compared to that on B. thuringiensis DB27 and S. aureus (Table 1B), to which P. pacificus is more resistant (Figure 1B).
Further, in P. pacificus, which is either more resistant to pathogens or shows slower mortality kinetics as compared to C. elegans, the expression profiles are observed to be usually smaller or just as large as that in C. elegans. We can rule out that these differences in profile size are due to potential differences in sensitivity of the two microarray platforms used, because we know from previous studies that our P. pacificus microarrays could detect differential expression of larger number of genes under different conditions such as dauer formation [22]. We also checked if changing the p-value cutoffs on microarray data abolishes the difference in profile sizes, but we find that the trend still holds (data not shown). Also, in C. elegans we have observed that when it is exposed to a non-pathogenic Bacillus subtilis strain for 4 hours, the number of genes differentially expressed is relatively low (∼510 genes) [II, AS and RJS, unpublished observations]. Hence, the differences in profile size between C. elegans and P. pacificus are most likely biologically relevant and not just a technical artifact.
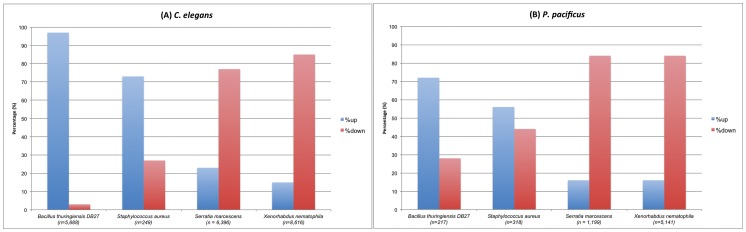
Gram-positive bacteria predominantly induce over-expression of genes while gram-negative bacteria cause transcriptional suppression
For both nematode species, the gram-positive bacteria tested induce up-regulation of relatively more genes as compared to down-regulation, while the reverse seems to be true for the gram-negative bacteria (Table 1). For example, in both the nematodes, exposure to the gram-positive pathogens B. thuringiensis DB27 and S. aureus causes induction of relatively more genes as compared to suppression (Figure 2, Table 1). On the other hand, exposure to the gram-negative pathogens S. marcescens and X. nematophila predominantly causes down-regulation of comparatively more genes than up-regulation (Figure 2, Table 1). Thus, the relative proportion of up-regulated versus down-regulated genes appears to depend upon some common factor(s) shared by either the gram-negative or the gram-positive bacteria, although more bacteria from both groups need to be tested to confirm this trend.
Figure 2. Gram-positive and gram-negative bacteria induce different proportions of up- versus down-regulated genes in C. elegans and P. pacificus.
Despite the differences in number of genes differentially expressed on each of the pathogens in the two nematodes, the relative proportion of up-regulated genes is higher than that of down-regulated genes on gram-positive bacteria B. thuringiensis and S. aureus in both (A) C. elegans and (B) P. pacificus. On the other hand, exposure to the gram-negative bacteria S. marcescens and X. nematophila results in down-regulation of a greater fraction of genes as compared to the up-regulated genes in both (A) C. elegans and (B) P. pacificus.
A bacterium-specific transcriptional response is mounted by both C. elegans and P. pacificus
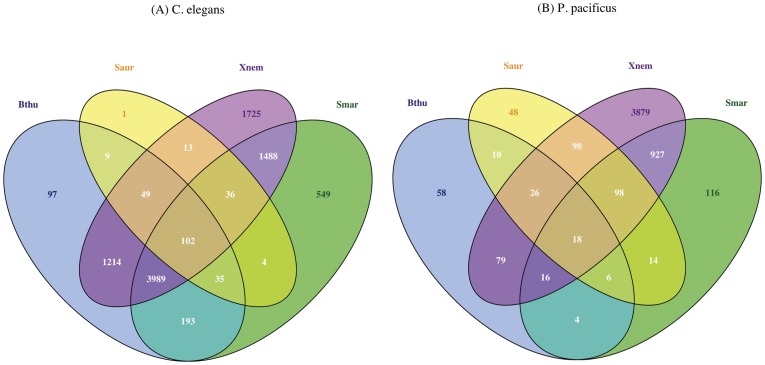
Next, we investigated the intra-specific response of the two nematodes after exposure to different pathogens. Although C. elegans is unable to survive on any of the four pathogens, we find that the expression profiles on each of the pathogens are qualitatively quite different from each other and only a small fraction of genes are common between expression profiles obtained on different pathogens (Figure 3A). Specifically, only 102 genes change their expression upon exposure to each of the four pathogens (Figure 3A, genes with nCommon = 4 in Table S1) but the number slightly increases to about 687 genes when the criterion is relaxed to significant differential expression in more than one expression profile (genes with nCommon >1 in Table S1). A small number of overlap between multiple pathogen response profiles is a signature for highly specific pathogen response, and has also been observed before e.g. only 22 genes were reported to be common between profiles after 24 hour exposure to Erwinia carotovara, Enterococcus faecalis, and Photorhabdus luminescens [46]. Interestingly, the genes induced in C. elegans across all four pathogens include the transcription factors pqm−1 and zip−2 (Table S1). The stress responsive transcription factor pqm−1 is also induced and required for defense in response to P. aeruginosa infection [43]. The bZIP transcription factor zip−2 is a known to regulate a subset of pmk-1 independent pathogen response genes on P. aeruginosa [15]. Similarly, the P. pacificus expression profiles also show a bacterium-specific signature, with only 18 genes being common across all the four profiles (n = 18, Figure 3B, genes with nCommon = 4 in Table S2) while 206 genes are common between the expression profiles on more than one pathogen (genes with nCommon >1 in Table S2).
Figure 3. A pathogen-specific transcriptional response is mounted by both C. elegans and P. pacificus upon exposure to different bacteria.
Overlap between the different genes differentially regulated in response to the four pathogens tested is represented as a Venn diagram for C. elegans and P. pacificus. Only 102 genes are found to be common between the expression profiles on all four pathogens in (A) C. elegans, while only 18 genes are common between the expression profiles corresponding to the four pathogens in (B) P. pacificus. This minimal overlap indicates the existence of a highly pathogen-specific immune response in both the nematodes. The abbreviations Bthu, Saur, Smar and Xnem refer to the bacteria Bacillus thuringiensis, Staphylococcus aureus, Serratia marcescens and Xenorhabdus nematophila respectively.
This pathogen specific nature of expression profiles within a nematode species is further highlighted in an expression cluster analysis (see Methods), where we compare our data-sets with various published microarray studies of pathogen-response [12], [43]–[50]. Based on the significance of overlaps between different microarray data sets (Table S3A and Table S4A for C. elegans and P. pacificus respectively), it is evident that only a small proportion of genes in each expression profile show an overlap with expression profiles on other pathogens.
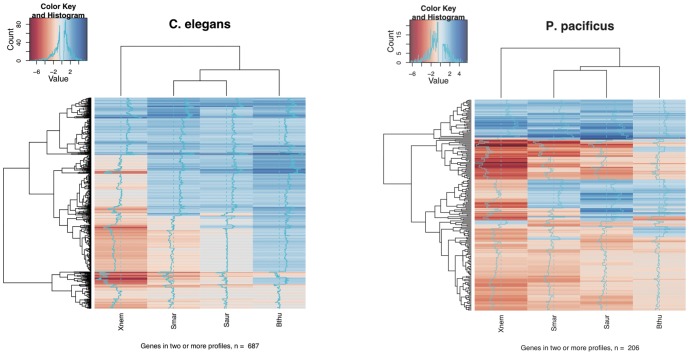
We further evaluated the extent of similarities between different intra-specific expression profiles, by carrying out a two-dimensional hierarchical clustering [51] on log-fold change values of genes that were significantly differentially expressed on at least two bacterium (n = 687 genes in C. elegans, and n = 206 genes in P. pacificus, number of common 1∶1 orthologs = 15, Table S1 and Table S2). Interestingly, in both C. elegans and P. pacificus heat-maps (Figure 4), the expression profile of X. nematophila response clusters separately from the profiles obtained in response to the other three bacteria, suggesting some differences in its pathogenicity mechanism(s) compared to other three bacteria. In summary, both C. elegans and P. pacificus show a bacterium-specific transcriptional response, with relatively few common genes being regulated across multiple bacteria in a given nematode.
Figure 4. Hierarchical clustering of genes differentially expressed on more than one pathogen identifies clusters of co-regulated genes.
The pathogen-response expression profiles for each nematode were clustered based on log2 of fold-changes for genes that were differentially expressed on at least two pathogens. In both (A) C. elegans and (B) P. pacificus, the expression profile in response to X. nematophila clusters separately from that in response to other pathogens, suggesting a difference in its mode of virulence from other pathogens.
Pfam domain enrichment analysis identifies a role for lipid metabolism and the detoxification machinery in pathogen response in P. pacificus
Identification of functional components in large gene-sets such as the pathogen response expression profiles can be better achieved by meta-analysis based on functional annotations rather than by searching for a gene-to-gene correspondence. Therefore, we tested for enrichment of various Pfam domains [52] in the proteins corresponding to the differentially expressed genes (see Methods for details) to see if common functional themes emerge despite only partially overlapping gene lists.
For C. elegans, we find the domain enrichment profile to be similar for all bacteria except S. aureus. The common domains include those related to Proteasome function, ATPase activity (AAA domain), DNA helicases with DEAD box and Helicase_C domains, and the RRM_1 motif that is indicative of RNA binding protein activity (Table S5). Thus even though the gene-by-gene similarity is low between these expression profiles, we observe proteins with similar functional domains to be enriched in all of them. In P. pacificus, expression profiles for all four bacteria are enriched for various lipid metabolism related domains such as Lipase_GDSL, FA_desaturase, Acyl-CoA_dh_1 and Abhydro_lipase (Table S6), suggesting a role for lipid metabolism in P. pacificus immune response. Studies on C. elegans immune response have shown that the poly-unsaturated fatty acids gamma-linolenic acid and stearidonic acid are integral for immune response, acting via the p38 MAP Kinase pathway [53]. Lipases can potentially function as antagonists of invading pathogens [54] and are known to be induced in response to pathogens in both C. elegans [9] and Drosophila melanogaster [55], [56]. We also see induction of lipase-like genes lipl-1 and lipl-3 across all pathogens in C. elegans (Table S1). Thus the enrichment of proteins containing lipase and related domains might contribute towards enhanced resistance of P. pacificus on some of the pathogens.
C- type lectins have been implicated in the C. elegans innate immune response [57] and in transcriptomic studies of exposure to P. aeruginosa, M. nematophila and S. marcescens [9], [43]–[46], [58]. Although C-type lectin domain encoding genes were differentially expressed when either C. elegans or P. pacificus was fed our four pathogens, the enrichment for the corresponding Pfam domain “Lectin_C” achieved statistical significance only in P. pacificus profiles on all pathogens except for that on S. aureus (Table S6).
The Pfam domains enriched in P. pacificus upon response to the relatively less pathogenic bacteria S. aureus as well as on exposure to the highly pathogenic X. nematophila also include various domains involved in detoxification and xenobiotic defense, such as Glucuronosyltransferase (UDPGT), Glutathione S-transferase (GST_C) and Cytochrome P450 domain (Table S6), which have been previously identified in expression studies of C. elegans exposed to xenobiotic compounds [59]. Interestingly, these domains have undergone an expansion in P. pacificus genome relative to the C. elegans genome and have been hypothesized to have adaptive significance in context of its necromenic lifestyle [18]. Here for the first time we show that the gene activity for the proteins containing these domains is enriched in a potentially pathogenic scenario and possibly confers an adaptive advantage.
Taken together, the Pfam domain analysis provides further insights into the pathogen response of the two nematodes. We observe similar Pfam domains to be enriched within a given nematode in response to different pathogens, but the set of enriched domains differs between C. elegans and P. pacificus, such that apart from the DNA helicase domain Helicase_C, we hardly find any other domains common between C. elegans and P. pacificus, even in response to the same bacteria.
Inhibition of translation machinery is a conserved effect of exposure to gram-negative pathogens in both C. elegans and P. pacificus
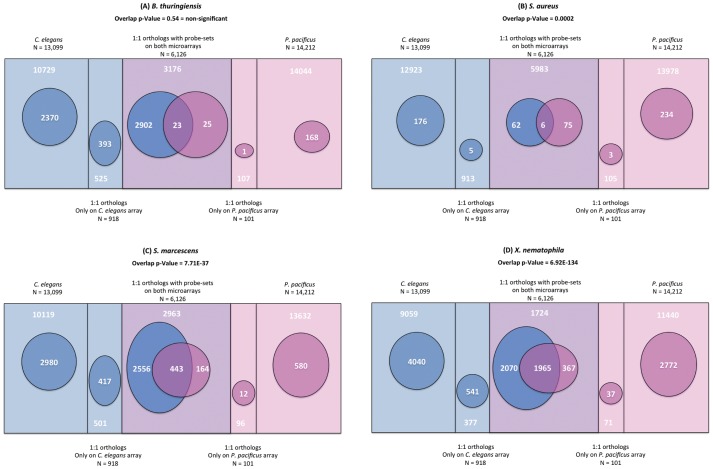
Since C. elegans and P. pacificus have very different survival behavior on the bacteria tested, we wanted to identify genes whose expression might be responsible for these differences. For comparing the expression profiles across the two nematode species, we restricted our analysis to the 6,126 1∶1 orthologous pairs defined by the stringent best reverse BLAST method, for which the probes were present on both the microarrays (see Methods). Interestingly, we see different patterns of overlap between the expression profiles of the two nematodes depending on the bacteria tested. For the gram-positive bacteria B. thuringiensis DB27 and S. aureus, which kill C. elegans at a much higher rate than P. pacificus, we observe a very limited overlap in the expression profiles of the two species (Figure 5A and Figure 5B). It was a priori not clear whether the P. pacificus resistance to B. thuringiensis and S. aureus is due to induction of similar genes as in C. elegans, albeit at higher expression levels, or, if the activation of a totally different set of genes causes the resistance phenotype. The surprisingly small extent of overlap observed in our comparisons supports the second model. Since most of the genes induced in P. pacificus on gram-positive bacteria do not have a characterized function yet, future studies will shed light on their role in innate immunity.
Figure 5. Overlap between C. elegans and P. pacificus expression profiles in response to different bacteria.
The rectangular boxes represent the entire transcriptomes of C. elegans and P. pacificus genes assayed on our microarrays, and their area of overlap represents the set of 6,126 1∶1 orthologs present on microarrays of both the nematodes. The ovals represent the fraction of differentially expressed genes in each of the subsets. For the 1∶1 orthologs, we assessed the significance of overlap between the genes differentially expressed in response to a given pathogen using a 2×2 Fisher's exact test. Differences or similarities in survival characteristics of the two nematodes when exposed to the same bacteria are reflected in their respective transcriptional responses. (A) On B. thuringiensis DB27, which is highly lethal to C. elegans but not to P. pacificus, only 23 genes are common between the respective expression profiles of the two nematodes, this overlap being statistically not significant. (B) Similarly, S. aureus is more lethal to C. elegans than P. pacificus, and the overlap between the corresponding expression profiles is limited to just 6 orthologs. Although this overlap is statistically significant (p-value = 0.0002), the extent of overlap is too small to be biologically significant. (C) S. marcescens is lethal to both the nematodes and the extent and significance overlap between the orthologs differentially expressed in the corresponding expression profiles is also relatively high (443 common orthologs, p-value = 7.71E–37). (D) On the pathogen X. nematophila, the observed overlap between the expression profiles in the two nematodes is even higher, with 2,093 orthologs regulated in both nematodes (p-value = 6.92E–134).
In contrast, we observe a highly significant overlap between the P. pacificus and C. elegans expression profiles in response to the gram-negative bacteria, S. marcescens and X. nematophila, which are lethal to both the nematodes (Figure 5C and Figure 5D). This suggests that either these gram-negative bacteria induce a similar immune response in both the nematodes, or that late and secondary markers of pathogenesis dominate the expression profile related damages in both the nematode species.
Exposure to pathogens can be expected to affect germline development and reproduction, which might contribute to the set of differentially expressed genes. Consistent with this expectation, we see enrichment of oocyte and germline related expression clusters [60], [61] in both C. elegans and P. pacificus profiles on various pathogens (see Tables S3 and S4, clusters “cgc6390:oogenesis-enriched”, “WBPaper00037611:RNP-8-associated” and “WBPaper00037611:GLD-2-associated”). The overlap is strongest with the genes downregulated in response to the most lethal pathogens S. marcescens and X. nematophila.
To identify the conserved elements of the pathogen response in the two species, we focused on the gram-negative pathogens S. marcescens and X. nematophila, which are lethal to both the nematodes. Almost all the genes common between the two nematodes (Figure 5C and Figure 5D) show a downregulation in response to each of these bacteria (Tables S7A and S7B). We further found 410 genes to be common across both two nematodes on both the gram-negative pathogens, most of them being downregulated (Table S7C). Interestingly, the stress responsive transcription factor encoded by pqm-1 was differentially expressed across all the four expression profiles (Table S7C), suggesting a potential and conserved role in innate immunity [43] across the two nematode species. Gene Ontology based analysis (Table S8) on any of these three lists shows an enrichment for biological processes related to “determination of adult lifespan”, as well as processes related to protein translation such as “translational elongation”, “translational initiation” and “ribosome biogenesis”. The corresponding terms under the ontology molecular function include “structural constituent of ribosome”, “translation elongation factor activity”, and “translation initiation factor activity”, and the enriched “cellular component” terms include “ribosome”, “ribonucleoprotein complex” and “small ribosomal unit”. Interestingly, it has recently been shown that inhibition of essential cellular processes like translation activates pathogen defense in C. elegans [62] and the gram-negative pathogen Pseudomonas aeruginosa response in C. elegans is activated by detecting its inhibitory effects on translation machinery [63], [64]. Downregulation of components of translational machinery and ribosomes was also observed upon germline-ablation of P. pacificus that exhibited an increase in life-span as well as resistance to the pathogen S. marcescens [66]. Hence our data together with these recent studies suggest that that downregulation of translation machinery could be a conserved response across the two nematodes, at least when exposed to gram-negative pathogens. We also find enrichment for genes involved in “proteasome complex” and “nuclear pore” complex, and these cellular components are known to have a potential role in immune response in C. elegans and P. pacificus longevity and immunity [62], [65], [66]. Other enriched processes commonly affected across the two nematodes include various processes related to metabolism, such as “glycolysis”, “tricarboxylic acid cycle” and “fatty acid metabolic process” (Table S8) and cellular compartment GO terms such as “mitochondrial membrane” and “mitochondrial proton-transporting ATP synthase complex” (Table S8). These results suggest that exposure to a pathogens leads to similar changes in the metabolic activity of the two nematodes.
It should also be noted that apart from the overlap between C. elegans and P. pacificus, differential expression of a substantial number of 1∶1 orthologs is specific to each of the nematode species. Additionally, the portion of the transcriptome with unresolved or no sequence similarity across the two species (Figure 5, rectangular areas specific to either C. elegans or P. pacificus) is also a significant contributor to the transcriptional response to the pathogens. Hence, based on these analysis of genes in the two nematodes exposed to the same gram-negative bacteria, it seems reasonable to conclude that some effectors of innate immunity are conserved across the two nematodes while some have diverged considerably during the last 250–300Mya separating the two nematode lineages.
Expression cluster based analysis identifies role for DAF-16, TGF-beta and p38 MAP Kinase pathways in pathogen response
To identify potential upstream regulators of immune response in the two nematodes, we tried using the existing knowledge from C. elegans to investigate what pathways appear to be mis-regulated. We therefore assessed the significance and the extent of overlap of our gene-sets with published microarray data sets available as “Expression Clusters” from WormBase [67] as well as with manually curated gene expression data from published microarray studies that were not available in WormBase (see Methods for details). These annotations were transferred to P. pacificus genes via the 1∶1 orthology relations.
In agreement with the role of DAF-16 in innate immunity [68], [69], we find DAF-16 targets to be enriched in genes up-regulated in C. elegans response to all pathogens except B. thuringiensis DB27 (cluster Murphy_etal_cgc5976_Class1 in Table S3B), while the DAF-16 repressed genes are over-represented in the set of down-regulated genes on B. thuringiensis and S. aureus (cluster Murphy_etal_cgc5976_Class2, Table S3B). Similarly, TGF-beta targets regulated by the ligand DBL-1 [70] are also enriched in many of our expression profiles (clusters “Roberts_etal_2010_DBL-1-UP” and “Roberts_etal_2010_DBL-1-DOWN”, Table S3B), confirming an important role of TGF-beta pathway in response to specific pathogens [9], .
Different MAP kinase pathways such as p38 MAPK and JNK pathways play a key role in C. elegans innate immunity and stress response [12], [49]. Consistent with this, we also see a significant overlap with genes regulated by the MAPKK SEK-1 and the JNK-like MAPK KGB-1, especially with down-regulated genes in all pathogen profiles (e.g cluster “Kao_etal2011_sek1_regulated”, Table S3B). We also observe a robust induction of starvation response genes [71] (e.g. cluster “WBPaper00032948:StarveUp3”, Table S3C) within just 4 hours of pathogen exposure, a reasonably short time not expected to induce actual starvation. This observation highlights the importance of metabolism related pathways in immune response [46], [72]–[75]. We also observe enrichment of various dauer related gene clusters and other clusters regulated by stress such as heat shock and oxidative stress in some of the C. elegans profiles (Table S3C).
Somewhat similar patterns of overlaps with C. elegans expression clusters are also seen for P. pacificus pathogen response profiles (Table S4). Interestingly, unlike C. elegans, the clusters of genes regulated in response to Cry5B toxin and KGB-1 or SEK-1 MAP kinases show a significant overlap only with genes down-regulated upon exposure of P. pacificus to S. marcescens and X. nematophila (e.g. cluster “Kao_etal2011_sek1_regulated”, Table S4B) but not with genes up-regulated in P. pacificus. This suggests potential differences either in targets of the MAPK pathways, or differences in mechanism of activation of these pathways, which might account for enhanced resistance of P. pacificus.
C. elegans DAF-16 targets are enriched in some P. pacificus profiles (cluster “Murphy_etal_cgc5976_Class2” in Table S4B), suggesting that DAF-16 might have a conserved role in innate response in both the species, at least on some pathogens. Interestingly, compared to C. elegans profiles, all P. pacificus profiles show a significant and more extensive overlap with genes involved in osmotic stress response [76] (e.g. cluster “WBPaper00035873:osmotically_regulated”, Table S4C), suggesting that osmotic regulation could be an important survival mechanism against potentially pathogenic bacteria [76].
Differential expression of P. pacificus pioneer genes
About 30% of the predicted transcriptome of P. pacificus is comprised of “pioneer genes”, which do not show any detectable homology to the known protein universe [19] and whose functions are not known. We investigated their potential role in pathogen response by looking at their expression data. We indeed find 832 of these pioneer genes to be differentially expressed P. pacificus in a pathogen specific manner, with 160 genes being regulated on at least two pathogens (Figure S3 and Table S9). On each of the pathogens, the pioneer genes constitute 12% to 18% of the active transcriptome, significantly less than the expected proportion of about 30% (Fisher's 2×2 exact test p –values<0.001, Figure S4). Interestingly however, we find the differential expression levels of these pioneer genes to be significantly higher than the non-pioneer fraction of the respective transcriptomes (Figures S5A, S5B, S5C and S5D, Kolmogorov-Smirnov test p-values less than 2.00E–16 for all four pathogens), indicating specific increase in their expression levels after exposure to pathogens. These observations together suggest that some of these lineage specific genes might have been acquired for adaptation to a microenvironment populated by different set of bacteria, some of which might be pathogenic. We can thus ascribe a putative role for these pioneer genes in pathogen response, although further studies will be needed to test these predictions.
An ecological perspective on the evolution of effectors and regulators of nematode immunity
Our finding that C. elegans mounts a pathogen-specific transcriptional response is in agreement with the current understanding in the field [46], [47], [58]. We further show for the first time that the nematode P. pacificus can also activate a pathogen-specific response. Many evolutionary mechanisms contribute towards generating this specificity in invertebrates, which lack an adaptive immune system. These include high genetic diversity receptors and effectors involved in pathogen recognition [77], evolutionary diversification of innate immunity effectors e.g. C-type lectins [57], lysozymes [78] and nlp- family of antimicrobial effectors [79], natural variation in host susceptibility and virulence of the pathogen [35], [38], [80], and evolution of mechanisms such as recombination and sexual reproduction [81] or alternative splicing [82], [83], all of which facilitate generation of genetic diversity.
The ecology of the organism is expected to be one of the key driving forces behind these changes, as the related species or even strains that occupy different ecological niches will be exposed to different set of non-pathogenic and pathogenic microbes and will need different strategies to survive. Due to these differences in selective pressures, the effectors of their immune systems can be expected to diverge rapidly and also affect the evolution of the host genomes.
The differences observed between response of C. elegans and P. pacificus can thus be best explained in the light of the distinct ecological niches occupied by both species. While C. elegans has recently been isolated from rotting fruit [28], Pristionchus nematodes and P. pacificus have a strong association with scarab beetles [29]–[32]. Once the beetle dies bacteria proliferate on the rotting carcass allowing mass growth of Pristionchus nematodes. Using a metagenomic approach we have previously shown that hundreds of plant and animal pathogenic bacteria occur on and in Pristionchus nematodes emerging from beetles [34]. Thus, Pristionchus is naturally exposed to a variety of bacteria and has evolved mechanisms to combat infections. Relative to C. elegans, the P. pacificus genome contains a larger set of genes encoding for cytochrome P450 and UDP-glucoronosyl/UDP-glucosyl transferases, which are required for coping with xenobiotic compounds [18], and we show here that differential activation of these gene families might contribute to its higher resistance to pathogens. The limited overlap on a gene-by-gene between expression profiles on different pathogens combined with the observation that similar Pfam domains are enriched within a nematode species, are consistent with evolutionary diversification and expansion of genes containing these functional domains.
Conclusions
This study provides a system wide analysis of the transcriptomic responses of the two nematode model species C. elegans and P. pacificus when feeding on four well-characterized bacterial pathogens. Studies on natural variation in the response of C. elegans to pathogens have contributed to micro-evolutionary studies of evolution of innate immunity. By adding studies on host-pathogen interactions in P. pacificus, we have tried to extend the evolution of innate immunity towards a macro-evolutionary perspective. We have studied nematode response as early as four hours after exposure to bacteria in order to capture initial events. While many previous studies have looked at various time points (4 h, 8 h, 24 h etc), it is known that by this time a common host necrotic response sets in [46]. The data generated for P. pacificus is the first of its kind, whereas our C. elegans dataset overlaps, in part, with previous studies. We performed these experiments de novo, rather than taking data from the literature, to rule out the effect of differences due to the microarray platform. Our C. elegans dataset is however, in strong agreement with existing datasets (e.g. [44], [46], [47]. This study fulfilled three major aims. First, it presents a platform for comparative systems biology analysis of two nematode model species. Second, it generates a catalog of genes involved in the evolution of nematode immunity and finally, it identifies pathogen-specific as well as pan-pathogen, conserved responses in the two nematode species.
Research on C. elegans and its interactions with bacteria has lead to the identification of several pathways involved in innate immunity [84]. By using an alternative nematode model we have expanded on this knowledge and shown conservation as well as divergence in the transcripts regulated during an immune response when fed different pathogens. Our systematic comparisons of nematode survival and gene expression on multiple pathogens highlights the fact that substantial differences exist in the repertoire of genetic components deployed in response to varied pathogens between C. elegans and P. pacificus. The resulting catalogs of pathogen specific and pan-pathogen genes provide an entry point to study the mechanism and evolution of individual response genes in future studies. Using expression cluster analysis we could show that homologs of known targets of FOXO/DAF-16, TGF-beta and p38 MAP kinase pathways in C. elegans are also significantly enriched in P. pacificus, suggesting that the key signaling pathways might regulate innate immunity in both the species. Given the lack of corresponding mutants in the relatively new model system P. pacificus, future studies are needed to test this hypothesis.
Evolutionary studies will have to involve more closely related species and strains given the strong differences observed for C. elegans and P. pacificus. From a P. pacificus perspective, more careful analyses of individual genes in additional strains and closely related Pristionchus species will be necessary to obtain insight into the evolution of immunity-related gens. Finally, these results argue for the importance of a comparative approach towards uncovering mechanistic details of the genetic basis that accounts for the cross-species variation in susceptibility to a given pathogen.
Materials and Methods
Strains
C. elegans N2 and P. pacificus RS2333 were maintained on NGM plates seeded with E. coli OP50. S. marcescens was isolated from La Reunion, S. aureus Newman was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Germany (DSMZ), X. nematophila was a gift from Becker Underwood, U.K. B. thuringiensis DB27 was isolated from dung beetles [35] and was initially thought to be a strain of B. cereus but further sequence analysis has shown it is in fact a strain of closely related B. thuringiensis.
Assessing survival of C. elegans and P. pacificus exposed to pathogens
Each bacterium was grown in a shaking incubator at 30°C overnight in LB, apart from S. aureus, which was grown at 37°C. The following day 100 μl were spread onto previously dried 6 cm NGM plates and incubated overnight. Twenty adults of C. elegans or P. pacificus were separately placed onto 3 NGM plates per bacterium and stored at 25°C where survival was monitored daily for 7 days. The experiment was repeated twice. Pathogen survival was compared to worms cultured on E. coli control plates and differences in survival were analyzed using Kaplan Meier and logrank test.
RNA collection for microarray experiments
Synchronized populations of C. elegans or P. pacificus were obtained by hypochlorite treatment and allowed to grow to young adult stage on E. coli OP50 plates at 20°C. For each biological replicate, about 200 young adult hermaphrodites were picked onto pathogen plates for 4 hours of pathogen exposure, after which they were collected into 1mL of TRIzol (Invitrogen). Equal number of corresponding age-matched control worms were exposed to E. coli OP50 for 4 hours and transferred to 1ml TRIzol (Invitrogen). Four biological replicates were collected for each experimental and control condition. Total RNA was extracted using TRIzol reagent (Invitrogen) according to manufacturer's instructions. The isolated RNA was further purified using phenol: chloroform: isoamyl alcohol precipitation to remove trace left-overs of TRIzol etc. which might interfere with downstream reactions. The RNA pellet was suspended in RNAse free water and was assessed on a Nanodrop spectrophotometer for quantity and RNA quality. RNA samples were stored at −80°C until the microarray experiments.
Microarray experiments
A total of 32 microarray hybridizations were carried out for 8 conditions (2 nematode species x 4 pathogenic bacteria; 4 biological replicates per condition). Oligonucleotide microarrays for C. elegans containing ∼43,000 unique probes for ∼20,000 C. elegans genes were obtained from Agilent Technologies (NCBI GEO accession GPL10094). For P. pacificus experiments, we used our custom designed oligonucleotide microarrays manufactured by Agilent Technologies, which contain ∼93,000 unique probes for the ∼23,000 P. pacificus predicted genes (NCBI GEO accession GPL14372, see [22] for design details of custom microarrays). The P. pacificus gene sequences are available at http://www.pristionchus.org/download/ and the gene models can be seen in the genome browser at http://www.pristionchus.org/cgi-bin/genome.pl.
Equal amounts of total RNA (500 ng to 800 ng) from four biological replicates of each experimental and control samples was used to produce Cy5 or Cy3 dye labeled cRNA using Quick Amp Labelling Kit (Agilent Technologies Inc., USA) as per manufacturer's instructions. Depending upon the amount of total RNA used, appropriate amounts of positive control RNA (Spike Mix-A and Spike Mix-B, from Agilent Technologies) was added to the mix before reverse transcribing the total RNA, as per manufacturer's instructions. We used the C. elegans or P. pacificus microarrays in a two-color format where Cy5 and Cy3 dye labeled cRNA from experimental and control sample is co-hybridized on the same microarray. The four biological replicates per experiment included two dye-swaps experiments to account for differences in dye labeling. Hybridization and washing of the arrays was carried according to manufacturer-supplied protocol. The arrays were scanned on a GenePix 4000B Microarray Scanner, and raw data extracted using GenePix Pro sofware (version 6).
Microarray data analysis
We used the Bioconductor [85] package limma [86] for analysis of our microarray data. Array quality was checked for parameters such as uniform background and foreground intensities over the entire array. The raw signal was background corrected using the normexp method [87] and the arrays were then lowess normalized individually (“normalizeWithinArrays” option), with differential weights assigned to probes and to positive control spike-ins, which are expected to show no fold change [88]. This differential weighing of probes is particularly necessary to account for differences in differences in relative proportion of mRNA versus total RNA, and/or differences in the amount of RNA produced per worm under different experimental conditions. Without this differential weighing scheme, the fold change calculations can be erroneous [22], [42]. The weight parameters were optimized based on MA-plots such that spike-in controls show their expected fold change values. lmFit function was used to fit a linear model to probe intensities across arrays, differential expression was calculated by empirical Bayes method using the eBayes function [89], and control of FDR was employed as the multiple testing correction. MA-plots were also used as diagnostic to identify and remove outlier arrays before fold-change calculations, such that at least three biological replicates were used for each experiment. Genes with a FDR corrected p-value less or equal to than 0.05 and absolute log2 of fold change greater than 0.5 were called significantly differentially expressed. Further data analysis was carried out using custom scripts in Perl and the statistical package R. Venn diagrams were drawn using the R package VennDiagram [90]. Raw and processed data from all the experiments from this publication have been deposited in a MIAME compliant format [91] at NCBI's Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). The accession numbers for C. elegans data are GSE36413, GSE36493, GSE36499, GSE36501, and the accession numbers for P. pacificus data are GSE36517, GSE36519, GSE36521 and GSE36523.
Identifying 1∶1 orthologs between C. elegans and P. pacificus
We have previously used a pairwise best BLASTP strategy to identify 7,176 pairs of 1∶1 orthologs between the C. elegans and P. pacificus [22]. Briefly, all protein sequences from C. elegans were run as query versus the database of P. pacificus gene predictions and vice versa. Only hits with a BLAST score > = 50 bits were retained, and mutually best hits were identified as 1∶1 orthologs. Probes for 6,126 of these gene pairs exist on microarrays of both the species.
Pfam domain annotation and enrichment analysis
Pfam domain annotations for C. elegans and P. pacificus proteomes (WS220 and predicted proteins respectively) were the same as described before [22]. Basically, hits with a p-value cut-off of 0.001 in HMM searches using HMMer 3.0 [92] on Pfam release V23/4 [52] were used as domain annotations. Only the domains, for which minimum 5 protein coding genes were represented on each microarray, were used for further enrichment analysis. Statistical significance of enrichment of Pfam domains in each expression profile determined using a 2×2 Fishers exact test, at a FDR corrected p-value cut-off of 0.05.
Expression cluster enrichment analysis
We have used “expression cluster” annotations from WormBase [67] in interpretations of other microarray expression profiles [22]. The list of microarray expression profiles in which a given C. elegans gene is known to be differentially expressed can be extracted from the section “Expression Cluster” from the WormBase gene summary page for each gene. We retrieved all available expression clusters for C. elegans genes from the WormBase web site. We also compiled data from other gene expression studies in C. elegans which are relevant to pathogen response but for which the corresponding expression clusters were not available in the WormBase (viz. [12], [49], [70]) and named these clusters with a prefix based on first author's last name and year of publication of the research article, and included them in our analysis. We inferred expression clusters for P. pacificus based on the set of 1∶1 orthologs. P-values for expression cluster enrichment in each expression profile was computed with a 2×2 Fisher exact test. FDR corrected p-value cut-off of 0.05 was used as the significance threshold. The significance score was calculated as –log10 of the p-values and was set to zero to indicate non-significance when p-values was greater than 0.05.
Gene ontology analysis, prediction of signal peptide and antimicrobial activity
Gene ontology analysis (presented in Table S8) was done using Bioconductor tool topGO, using method “elimFisher” for calculating p-values [93]. For analyzing features of differentially expressed pioneer genes in P. pacificus (Table S9), SignalP tool was used to predict the presence of a signal peptide [94], and for genes coding for products smaller than 100 amino acids, CAMP tool [95] was used to predict whether they can act as potential Anti-Microbial Peptides (AMPs).
Supporting Information
Long-term survival curves for P. pacificus on various pathogens. P. pacificus has higher resistance than C. elegans, with longer median survival time of about 8 days on B. thuringiensis and S. aureus.
(PDF)
Global transcriptional suppression in response to pathogens. Exposure to pathogens resulted in a decrease of amount of total RNA produced per worm when compared to the non-pathogenic E. coli strain. This global decrease in transcription is seen in both (A) C. elegans and (B) P. pacificus.
(PDF)
Overlap between pioneer genes regulated in P. pacificus in response to the four pathogens. Of the 832 pioneer genes differentially expressed on any of the pathogens in P. pacificus, 160 genes are common between two or more than two expression profiles.
(PDF)
Relative proportions of pioneer genes versus non-pioneer genes in the active transcriptome of P. pacificus on each of the four pathogens. The P. pacificus genome contains about 30% pioneer genes. Compared to the random expectation of the same proportion of pioneer genes in different expression profiles, they are found to significantly under-represented (Fisher's test p-values<2E-16 for each comparison with whole-genome distribution.
(PDF)
Pioneer genes are expressed at higher levels than non-pioneer genes in each of the pathogen-induced expression profiles on P. pacificus . Cumulative distributions of fold-changes of pioneer genes (red curves) and non-pioneer genes (blue curves) for genes differentially expressed on (A) B. thuringiensis DB27 (B) S. aureus (C) S. marcescens and (D) X. nematophila. The Kolmogorov-Smirnov test p-values are less that 2E-16 in each case.
(PDF)
Differential expression of genes in C. elegans exposed to different pathogens.
(XLSX)
Differential expression of genes in P. pacificus exposed to different pathogens.
(XLSX)
Expression clusters enriched in genes up- and down-regulated in C. elegans upon exposure to each of the four pathogens.
(XLSX)
Expression clusters enriched in genes up- and down-regulated in P. pacificus upon exposure to each of the four pathogens.
(XLSX)
Pfam domains enriched in C. elegans expression profiles in response to different pathogens.
(XLSX)
Pfam domains enriched in P. pacificus expression profiles in response to different pathogens.
(XLSX)
Genes common across expression profiles of C. elegans and P. pacificus upon exposure to the gram-negative bacteria (A) S. marcescens (B) X. nematophila , and (C) common to both S. marcescens and X. nematophila expression profiles.
(XLSX)
GO enrichment analysis of genes common across both C. elegans and P. pacificus profiles obtained upon exposure to gram-negative bacteria S. marcescens and X. nematophila .
(XLSX)
Features of pioneer genes differentially expressed on any of the four pathogens in P. pacificus .
(XLSX)
Acknowledgments
We thank members of the lab for discussion and Dr. Mitch Levesque for help with the microarray platform.
Funding Statement
This work was funded by the Max-Planck Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haldane JBS (1949) Disease and Evolution. La Ricerca Scientifica 19: 68–76. [Google Scholar]
- 2.Thompson JN (1994) The coevolutionary process. University of Chicago Press. 398 p.
- 3. Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR (2002) Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet 32: 569–577 doi:10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- 4.Frank SA (2002) Immunology and Evolution of Infectious Disease. Princeton (NJ): Princeton University Press; Available: http://www.ncbi.nlm.nih.gov/books/NBK2394/. [PubMed]
- 5. Griffitts JS, Whitacre JL, Stevens DE, Aroian RV (2001) Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293: 860–864 doi:10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- 6. Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, et al. (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297: 623–626 doi:10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 7. Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA 96: 2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurz CL, Chauvet S, Andrès E, Aurouze M, Vallet I, et al. (2003) Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J 22: 1451–1460 doi:10.1093/emboj/cdg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, et al. (2002) Inducible Antibacterial Defense System in C. elegans. Current Biology 12: 1209–1214 doi:10.1016/S0960-9822(02)00928-4. [DOI] [PubMed] [Google Scholar]
- 10. Aballay A, Ausubel FM (2001) Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci USA 98: 2735–2739 doi:10.1073/pnas.041613098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, et al. (2003) Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300: 1921 doi:10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 12. Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, et al. (2004) Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci USA 101: 10995–11000 doi:10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicholas HR, Hodgkin J (2004) The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr Biol 14: 1256–1261 doi:10.1016/j.cub.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Powell JR, Kim DH, Ausubel FM (2009) The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proceedings of the National Academy of Sciences 106: 2782 –2787. doi:10.1073/pnas.0813048106. [DOI] [PMC free article] [PubMed]
- 15.Estes KA, Dunbar TL, Powell JR, Ausubel FM, Troemel ER (2010) bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proceedings of the National Academy of Sciences 107: 2153 –2158. doi:10.1073/pnas.0914643107. [DOI] [PMC free article] [PubMed]
- 16. Irazoqui JE, Ng A, Xavier RJ, Ausubel FM (2008) Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci USA 105: 17469–17474 doi:10.1073/pnas.0809527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong RL, Sommer RJ (2006) Pristionchus pacificus: a well-rounded nematode. Bioessays 28: 651–659 doi:10.1002/bies.20404. [DOI] [PubMed] [Google Scholar]
- 18. Dieterich C, Clifton SW, Schuster LN, Chinwalla A, Delehaunty K, et al. (2008) The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet 40: 1193–1198 doi:10.1038/ng.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borchert N, Dieterich C, Krug K, Schütz W, Jung S, et al. (2010) Proteogenomics of Pristionchus pacificus reveals distinct proteome structure of nematode models. Genome Res 20: 837–846 doi:10.1101/gr.103119.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pires da Silva A (2005) Pristionchus pacificus genetic protocols. WormBook: 1–8. doi:10.1895/wormbook.1.114.1. [DOI] [PMC free article] [PubMed]
- 21. Schlager B, Wang X, Braach G, Sommer RJ (2009) Molecular cloning of a dominant roller mutant and establishment of DNA-mediated transformation in the nematode Pristionchus pacificus. Genesis 47: 300–304 doi:10.1002/dvg.20499. [DOI] [PubMed] [Google Scholar]
- 22. Sinha A, Sommer RJ, Dieterich C (2012) Divergent gene expression in the conserved dauer stage of the nematodes Pristionchus pacificus and Caenorhabditis elegans. BMC Genomics 13: 254 doi:10.1186/1471-2164-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian H, Schlager B, Xiao H, Sommer RJ (2008) Wnt signaling induces vulva development in the nematode Pristionchus pacificus. Curr Biol 18: 142–146 doi:S0960–9822(07)02479–7. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Sommer RJ (2011) Antagonism of LIN-17/Frizzled and LIN-18/Ryk in Nematode Vulva Induction Reveals Evolutionary Alterations in Core Developmental Pathways. PLoS Biol 9: e1001110 doi:10.1371/journal.pbio.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudel D, Riebesell M, Sommer RJ (2005) Gonadogenesis in Pristionchus pacificus and organ evolution: development, adult morphology and cell-cell interactions in the hermaphrodite gonad. Dev Biol 277: 200–221 doi:S0012-1606(04)00658-X. [DOI] [PubMed] [Google Scholar]
- 26. Pires-daSilva A, Sommer RJ (2004) Conservation of the global sex determination gene tra-1 in distantly related nematodes. Genes Dev 18: 1198–1208 doi:15155582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong RL, Witte H, Sommer RJ (2008) Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL–4. Proc Natl Acad Sci USA 105: 7779–7784 doi:0708406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Félix M-A, Braendle C (2010) The natural history of Caenorhabditis elegans. Curr Biol 20: R965–969 doi:10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 29. Herrmann M, Mayer WE, Sommer RJ (2006) Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology (Jena) 109: 96–108 doi:S0944-2006(06)00006-7. [DOI] [PubMed] [Google Scholar]
- 30. Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, et al. (2007) The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zool Sci 24: 883–889 doi:0289-0003-24-9-883. [DOI] [PubMed] [Google Scholar]
- 31. Herrmann M, Kienle S, Rochat J, Mayer WE, Sommer RJ (2010) Haplotype diversity of the nematode Pristionchus pacificus on Réunion in the Indian Ocean suggests multiple independent invasions. Biological Journal of the Linnean Society 100: 170–179 doi:10.1111/j.1095-8312.2010.01410.x. [Google Scholar]
- 32. Morgan K, McGaughran A, Villate L, Herrmann M, Witte H, et al. (2012) Multi locus analysis of Pristionchus pacificus on La Réunion Island reveals an evolutionary history shaped by multiple introductions, constrained dispersal events and rare out-crossing. Mol Ecol 21: 250–266 doi:10.1111/j.1365-294X.2011.05382.x. [DOI] [PubMed] [Google Scholar]
- 33. Wei J-Z, Hale K, Carta L, Platzer E, Wong C, et al. (2003) Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci USA 100: 2760–2765 doi:10.1073/pnas.0538072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, et al. (2008) Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J Exp Biol 211: 1927–1936 doi:211/12/1927. [DOI] [PubMed] [Google Scholar]
- 35. Rae R, Iatsenko I, Witte H, Sommer RJ (2010) A subset of naturally isolated Bacillus strains show extreme virulence to the free-living nematodes Caenorhabditis elegans and Pristionchus pacificus. Environ Microbiol 12: 3007–3021 doi:10.1111/j.1462-2920.2010.02278.x. [DOI] [PubMed] [Google Scholar]
- 36.Altun, Z.F., Herndon, L.A., Crocker, C., Lints, R. and Hall, D.H. (ed.s) (2002–2012) WormAtlas. Available: http://www.wormatlas.org.
- 37. Sommer RJ, Carta LK, Kim SY, Sternberg PW (1996) Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda: Neodiplogastridae). Fundamental and Applied Nematology 19: 511–522. [Google Scholar]
- 38. Schulenburg H, Ewbank JJ (2004) Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol Biol 4: 49 doi:10.1186/1471-2148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forst S, Clarke D (2002) Bacteria-Nematode Symbiosis. In: Gaugler R, editor. Entomopathogenic nematology. CABI. 57–78.
- 40. Hsin H, Kenyon C (1999) Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399: 362–366 doi:10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 41. Dalley BK, Golomb M (1992) Gene expression in the Caenorhabditis elegans dauer larva: developmental regulation of Hsp90 and other genes. Dev Biol 151: 80–90. [DOI] [PubMed] [Google Scholar]
- 42. van de Peppel J, Kemmeren P, van Bakel H, Radonjic M, van Leenen D, et al. (2003) Monitoring global messenger RNA changes in externally controlled microarray experiments. EMBO Rep 4: 387–393 doi:10.1038/sj.embor.embor798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shapira M, Brigham BJ, Rong J, Chen K, Ronen M, et al. (2006) A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proceedings of the National Academy of Sciences 103: 14086–14091 doi:10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, et al. (2006) p38 MAPK Regulates Expression of Immune Response Genes and Contributes to Longevity in C. elegans. PLoS Genet 2: e183 doi:10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Rourke D, Baban D, Demidova M, Mott R, Hodgkin J (2006) Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res 16: 1005–1016 doi:10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank J (2007) Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biology 8: R194 doi:10.1186/gb-2007-8-9-r194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Irazoqui JE, Troemel ER, Feinbaum RL, Luhachack LG, Cezairliyan BO, et al. (2010) Distinct Pathogenesis and Host Responses during Infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog 6: e1000982 doi:10.1371/journal.ppat.1000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bolz DD, Tenor JL, Aballay A (2010) A conserved PMK-1/p38 MAPK is required in caenorhabditis elegans tissue-specific immune response to Yersinia pestis infection. J Biol Chem 285: 10832–10840 doi:10.1074/jbc.M109.091629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kao C-Y, Los FCO, Huffman DL, Wachi S, Kloft N, et al. (2011) Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog 7: e1001314 doi:10.1371/journal.ppat.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pukkila-Worley R, Ausubel FM, Mylonakis E (2011) Candida albicans Infection of Caenorhabditis elegans Induces Antifungal Immune Defenses. PLoS Pathog 7: e1002074 doi:10.1371/journal.ppat.1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. PNAS 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finn RD, Mistry J, Tate J, Coggill P, Heger A, et al. (2009) The Pfam protein families database. Nucleic Acids Research 38: D211–D222 doi:10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nandakumar M, Tan M-W (2008) Gamma-linolenic and stearidonic acids are required for basal immunity in Caenorhabditis elegans through their effects on p38 MAP kinase activity. PLoS Genet 4: e1000273 doi:10.1371/journal.pgen.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schulenburg H, Kurz CL, Ewbank JJ (2004) Evolution of the innate immune system: the worm perspective. Immunol Rev 198: 36–58. [DOI] [PubMed] [Google Scholar]
- 55. De Gregorio E, Spellman PT, Rubin GM, Lemaitre B (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA 98: 12590–12595 doi:10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, et al. (2001) A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci USA 98: 15119–15124 doi:10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schulenburg H, Hoeppner MP, Weiner J III, Bornberg-Bauer E (2008) Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiology 213: 237–250 doi:10.1016/j.imbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 58. Engelmann I, Griffon A, Tichit L, Montañana-Sanchis F, Wang G, et al. (2011) A Comprehensive Analysis of Gene Expression Changes Provoked by Bacterial and Fungal Infection in C. elegans. PLoS ONE 6: e19055 doi:10.1371/journal.pone.0019055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reichert K, Menzel R (2005) Expression profiling of five different xenobiotics using a Caenorhabditis elegans whole genome microarray. Chemosphere 61: 229–237 doi:10.1016/j.chemosphere.2005.01.077. [DOI] [PubMed] [Google Scholar]
- 60. Reinke V, Gil IS, Ward S, Kazmer K (2004) Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323 doi:10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 61. Kim KW, Wilson TL, Kimble J (2010) GLD-2/RNP-8 cytoplasmic poly(A) polymerase is a broad-spectrum regulator of the oogenesis program. Proc Natl Acad Sci USA 107: 17445–17450 doi:10.1073/pnas.1012611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Melo JA, Ruvkun G (2012) Inactivation of Conserved C. elegans Genes Engages Pathogen- and Xenobiotic-Associated Defenses. Cell 149: 452–466 doi:10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McEwan DL, Kirienko NV, Ausubel FM (2012) Host Translational Inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an Immune Response in Caenorhabditis elegans. Cell Host Microbe 11: 364–374 doi:10.1016/j.chom.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dunbar TL, Yan Z, Balla KM, Smelkinson MG, Troemel ER (2012) C. elegans Detects Pathogen-Induced Translational Inhibition to Activate Immune Signaling. Cell Host Microbe 11: 375–386 doi:10.1016/j.chom.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alper S, Laws R, Lackford B, Boyd WA, Dunlap P, et al. (2008) Identification of innate immunity genes and pathways using a comparative genomics approach. Proc Natl Acad Sci USA 105: 7016–7021 doi:10.1073/pnas.0802405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rae R, Sinha A, Sommer RJ (2012) Genome-Wide Analysis of Germline Signaling Genes Regulating Longevity and Innate Immunity in the Nematode Pristionchus pacificus. PLoS Pathogens 8: e1002864 doi:10.1371/journal.ppat.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harris TW, Antoshechkin I, Bieri T, Blasiar D, Chan J, et al. (2010) WormBase: a comprehensive resource for nematode research. Nucleic Acids Res 38: D463–467 doi:10.1093/nar/gkp952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283 doi:10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 69. Lee SS, Kennedy S, Tolonen AC, Ruvkun G (2003) DAF-16 Target Genes That Control C. elegans Life-Span and Metabolism. Science 300: 644–647 doi:10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 70. Roberts AF, Gumienny TL, Gleason RJ, Wang H, Padgett RW (2010) Regulation of genes affecting body size and innate immunity by the DBL-1/BMP-like pathway in Caenorhabditis elegans. BMC Dev Biol 10: 61 doi:10.1186/1471-213X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Baugh LR, Demodena J, Sternberg PW (2009) RNA Pol II accumulates at promoters of growth genes during developmental arrest. Science 324: 92–94 doi:10.1126/science.1169628. [DOI] [PubMed] [Google Scholar]
- 72. Coolon JD, Jones KL, Todd TC, Carr BC, Herman MA (2009) Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. PLoS Genet 5: e1000503 doi:10.1371/journal.pgen.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anagnostou SH, Shepherd PR (2008) Glucose induces an autocrine activation of the Wnt/beta-catenin pathway in macrophage cell lines. Biochem J 416: 211–218 doi:10.1042/BJ20081426. [DOI] [PubMed] [Google Scholar]
- 74. Bensinger SJ, Tontonoz P (2008) Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454: 470–477 doi:10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 75. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, et al. (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107 doi:10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rohlfing A-K, Miteva Y, Hannenhalli S, Lamitina T (2010) Genetic and Physiological Activation of Osmosensitive Gene Expression Mimics Transcriptional Signatures of Pathogen Infection in C. elegans. PLoS ONE 5: e9010 doi:10.1371/journal.pone.0009010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schulenburg H, Boehnisch C, Michiels NK (2007) How do invertebrates generate a highly specific innate immune response? Mol Immunol 44: 3338–3344 doi:10.1016/j.molimm.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 78. Schulenburg H, Boehnisch C (2008) Diversification and adaptive sequence evolution of Caenorhabditis lysozymes (Nematoda: Rhabditidae). BMC Evol Biol 8: 114 doi:10.1186/1471-2148-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, et al. (2008) Anti-Fungal Innate Immunity in C. elegans Is Enhanced by Evolutionary Diversification of Antimicrobial Peptides. PLoS Pathog 4: e1000105 doi:10.1371/journal. at.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Félix M-A, Ashe A, Piffaretti J, Wu G, Nuez I, et al. (2011) Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol 9: e1000586 doi:10.1371/journal.pbio.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hamilton WD, Axelrod R, Tanese R (1990) Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci USA 87: 3566–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Watson FL, Püttmann-Holgado R, Thomas F, Lamar DL, Hughes M, et al. (2005) Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309: 1874–1878 doi:10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 83. Dong Y, Taylor HE, Dimopoulos G (2006) AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol 4: e229 doi:10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ewbank JJ (2006) Signaling in the immune response. WormBook. doi: 10.1895/wormbook.1.83.1 [DOI] [PMC free article] [PubMed]
- 85. Gentleman R, Carey V, Bates D, Bolstad B, Dettling M, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biology 5: R80 doi:10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smyth GK (2005) Limma: linear models for microarray data. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer, New York. 397–420.
- 87. Ritchie ME, Silver J, Oshlack A, Holmes M, Diyagama D, et al. (2007) A comparison of background correction methods for two-colour microarrays. Bioinformatics 23: 2700–2707 doi:10.1093/bioinformatics/btm412. [DOI] [PubMed] [Google Scholar]
- 88. Smyth GK, Speed T (2003) Normalization of cDNA microarray data. Methods 31: 265–273 doi:10.1016/S1046-023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 89.Smyth GK (2004) Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Statistical Applications in Genetics and Molecular Biology 3. doi:10.2202/1544-6115.1027. [DOI] [PubMed]
- 90. Chen H, Boutros P (2011) VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 12: 35 doi:10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, et al. (2001) Minimum information about a microarray experiment (MIAME) – toward standards for microarray data. Nat Genet 29: 365–371 doi:10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 92. Eddy SR (2009) A new generation of homology search tools based on probabilistic inference. Genome Inform 23: 205–211. [PubMed] [Google Scholar]
- 93. Alexa A, Rahnenfuhrer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 doi:10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- 94. Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786 doi:10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 95. Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula-Thomas S (2010) CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res 38: D774–780 doi:10.1093/nar/gkp1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long-term survival curves for P. pacificus on various pathogens. P. pacificus has higher resistance than C. elegans, with longer median survival time of about 8 days on B. thuringiensis and S. aureus.
(PDF)
Global transcriptional suppression in response to pathogens. Exposure to pathogens resulted in a decrease of amount of total RNA produced per worm when compared to the non-pathogenic E. coli strain. This global decrease in transcription is seen in both (A) C. elegans and (B) P. pacificus.
(PDF)
Overlap between pioneer genes regulated in P. pacificus in response to the four pathogens. Of the 832 pioneer genes differentially expressed on any of the pathogens in P. pacificus, 160 genes are common between two or more than two expression profiles.
(PDF)
Relative proportions of pioneer genes versus non-pioneer genes in the active transcriptome of P. pacificus on each of the four pathogens. The P. pacificus genome contains about 30% pioneer genes. Compared to the random expectation of the same proportion of pioneer genes in different expression profiles, they are found to significantly under-represented (Fisher's test p-values<2E-16 for each comparison with whole-genome distribution.
(PDF)
Pioneer genes are expressed at higher levels than non-pioneer genes in each of the pathogen-induced expression profiles on P. pacificus . Cumulative distributions of fold-changes of pioneer genes (red curves) and non-pioneer genes (blue curves) for genes differentially expressed on (A) B. thuringiensis DB27 (B) S. aureus (C) S. marcescens and (D) X. nematophila. The Kolmogorov-Smirnov test p-values are less that 2E-16 in each case.
(PDF)
Differential expression of genes in C. elegans exposed to different pathogens.
(XLSX)
Differential expression of genes in P. pacificus exposed to different pathogens.
(XLSX)
Expression clusters enriched in genes up- and down-regulated in C. elegans upon exposure to each of the four pathogens.
(XLSX)
Expression clusters enriched in genes up- and down-regulated in P. pacificus upon exposure to each of the four pathogens.
(XLSX)
Pfam domains enriched in C. elegans expression profiles in response to different pathogens.
(XLSX)
Pfam domains enriched in P. pacificus expression profiles in response to different pathogens.
(XLSX)
Genes common across expression profiles of C. elegans and P. pacificus upon exposure to the gram-negative bacteria (A) S. marcescens (B) X. nematophila , and (C) common to both S. marcescens and X. nematophila expression profiles.
(XLSX)
GO enrichment analysis of genes common across both C. elegans and P. pacificus profiles obtained upon exposure to gram-negative bacteria S. marcescens and X. nematophila .
(XLSX)
Features of pioneer genes differentially expressed on any of the four pathogens in P. pacificus .
(XLSX)