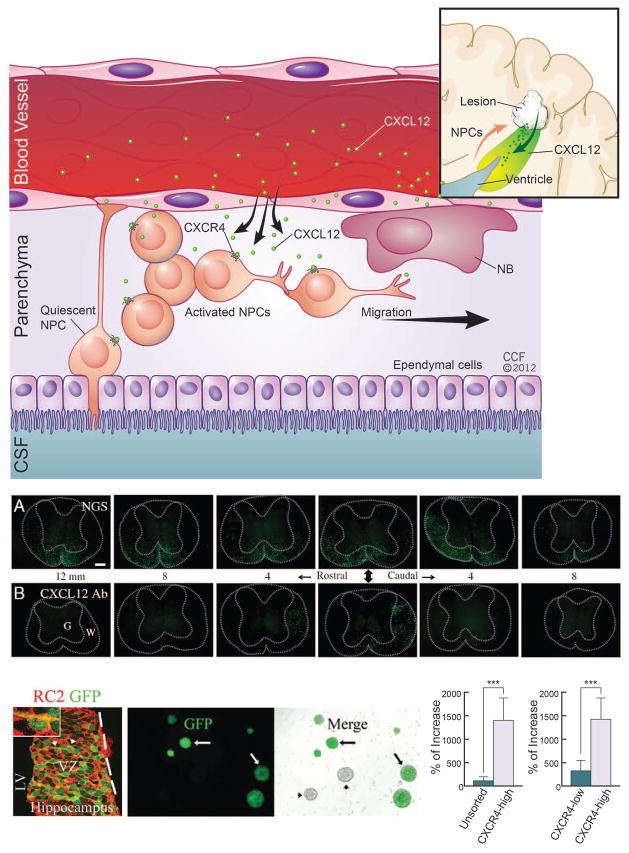
Figure 2. CXCL12 signaling in the SVZ.
(a) A stem cell niche in the SVZ is required to maintain the self-renewal and multipotency of neural stem cells (NSCs). The perivascular space provides a microenvironment to influence the normal roles of NSCs for adult neurogenesis. As suggested in previous studies [9], vessels secrete CXCL12 and regulate the activation of NSCs, which constitutively express CXCR4 receptors. In this hypothesis, NSCs are transformed from an original quiescent state to an activated state. Activated NSCs partially differentiate into neural progenitor cells (NPCs). In addition, vascular niches can be newly generated within damage tissue in many neurodegenerative diseases (inset). These pathologically-associated vascular niches may potentially provide inducible CXCL12 (green arrow) and form a gradient ligand concentration (colored by green) to recruit NPCs or neuronal blast cells (NBs) to enhance stem cell-based tissue repair (red arrow). (b) CXCL12 is critical for NSC engraftment in the spinal cord in a JHM strain of mouse hepatitis virus (JHMV) mouse model. NSC engraftment, as visualized by genetically-labeled green fluorescent protein (GFP) reporter, was observed to be reduced in the presence of a CXCL12 blocking antibody (CXCL12 Ab; bottom panels) as compared to a protein control (normal goat serum, NGS; top panels) [96]. Starting from implanted sites (middle panels pointed by arrow heads), GFP-NSCs migrate toward both rostral and caudal spinal cords. Each panel has 4μM distance. (c) CXCR4 is expressed in early NSCs in vitro and in vivo as demonstrated using a CXCR4-GFP transgenic mouse model. Panel i: GFP-NSCs co-express an early radial cell marker RC2 (clone RC2). Panels ii-iii: GFP-NSCs can be cultured and visualized under fluorescent microscopy. Panels iv-v: GFP-NSCs can be enriched via flow cytometry-based cell sorting (FACS) for sphere formation [11]. CSF (cerebrospinal fluid). Adapted, with permission, from [96] (b) and [11] (c).