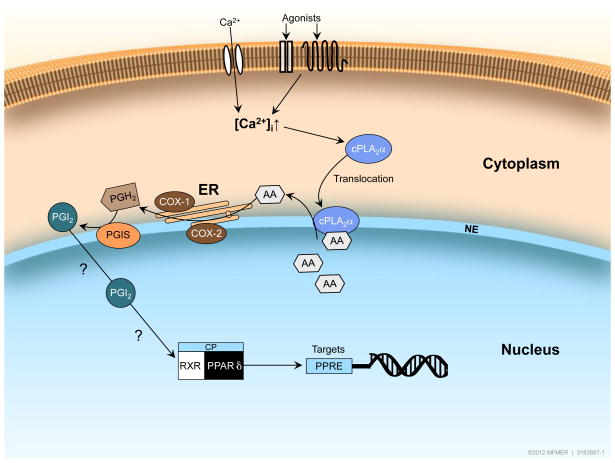
Figure 1.
Hypothetical model of the cellular localization of enzymes responsible for PGI2 synthesis and subsequent activation of PPARδ. Increases in intracellular Ca2+ levels cause activation and translocation of cPLA2α from the cytoplasm to the nuclear envelope. (Complexity of cPLA2α activation has been simplified to include only activation by increased Ca2+ levels). Mobilization of arachidonic acid from phospholipids by cPLA2α provides substrate for enzyme activity of COX-1 or COX-2 and PGIS resulting in production of PGI2 and subsequent activation of nuclear receptor PPARδ. PPARδ-RXR heterodimers undergo a conformational shift and this causes dismissal of the co-repressor complex in exchange for co-activator proteins thereby resulting in enhanced PPARδ target gene expression. Exact nature of agonists and physical stimuli responsible for PGI2-induced activation of PPARδ remains to be determined (denoted by ? in the figure). cPLA2=cytosolic phospholipase A2; ER = endoplasmic reticulum; NE = nuclear envelope; AA = arachidonic acid; PGI2 = prostacyclin; PGH2 = prostaglandin H2; PGIS = prostacyclin synthase; RXR = retinoid X receptor; CP = co-activator proteins; PPRE = PPARδ responsive element.