Abstract
Here, we report an improved method to analyze antioxidant activity using the europium tetracycline assay developed by Duerkop and Wolfbeis (J Fluor 15(5):755–761, 2005). The europium tetracycline hydrogen peroxide reduction assay (EHRA) accurately measures antioxidant activity based on hydrogen peroxide scavenging. Several known antioxidant compounds were assessed with the EHRA and a stoichiometric relationship between the number of oxidant molecules trapped per molecule of antioxidant was identified. Various extracts of hops were also tested to validate this method for use with natural extracts; water extraction yielded the highest level of antioxidant activity. Hop leaves were shown to be a better source of antioxidants relative to the traditional hop cones. The data also indicate that the EHRA may serve to breach the hydrophilic/lipophilic gap in antioxidant screening as the europium tetracycline probe is effective in many solvents. The EHRA thus provides a robust and inexpensive measure of antioxidant activity.
Keywords: Europium tetracycline, EHRA, ROS, Hops, Hydrogen peroxide, Antioxidant assay
Introduction
Reactive oxygen species (ROS) are a natural part of life and are produced during aerobic growth due to the incomplete reduction of oxygen [15, 30]. In addition, ROS may also be produced following damage by ultraviolet (UV) light or ionizing radiation. Various species of ROS have been found to affect cellular function by impairing enzyme activity, altering membrane permeability and damaging DNA [9, 19, 20]. Hydroperoxides are one of the major forms of ROS which impact both cells and cellular environments alike. ROS have also gained attention in the food and beverage industry as they have been identified as key factors in food spoilage [13]. Hydrogen peroxide (H2O2) was determined to be the key ROS which caused staling during the fermentation and maturation of lager beer [25, 43, 44, 50]. Prevention of early oxidation of beer during production has been speculated to greatly increase subsequent shelf life, flavor, and aroma characteristics [25, 45].
A variety of natural compounds counteract these deleterious oxidants. These “antioxidants” include simple small molecules such as sulfur dioxide as well as specialized enzymes such as catalase or superoxide dismutase. Accordingly, various techniques have been developed to measure antioxidant activity. Free radical trapping reagents such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) [2], 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) [4, 33], and the radical initiator 2,2′-azino-bis(2-amidinopropane) didryochloride [5, 27, 35] have been used for many years. Various other approaches have also been employed, including chemiluminescent methods involving luminol [46], electron spin resonance [22, 23], and enzymatic inhibition [39]. The majority of these tests are either expensive or time-consuming and often do not specifically test for biologically relevant oxidants; the oxidant assessed is often generated by an enzyme or a nonphysiological radical source. In addition, the antioxidant–oxidant network is difficult to determine as there are many pathways for assay inhibition, including interaction with the probe, enzyme inhibition, and signal quenching through interferents such as free ions.
Use of the europium tetracycline (EuTc) complex is a novel approach to addressing these issues. EuTc is a lanthanide-based fluorescent probe that has previously been used to detect H2O2 [40], urea hydrogen peroxide [7], phosphate [11], glucose [47], and for catalase and peroxidase activity assays [48]. Original studies by Rakicioglu et al. [40] showed that H2O2 can cause a measurable increase in the fluorescence of EuTc at 616 nm. Further studies by Durkop and Wolfbeis [12] sought to optimize the assay conditions and EuTc fluorescence finding that a near-neutral pH gave an intense level of fluorescence and the most significant difference between bound and unbound hydrogen peroxide. The proposed mechanism of interaction between the EuTc complex and hydrogen peroxide involves the displacement of water molecules bonded to Eu3+ to form a new ligand without undergoing any significant redox reactions. The replacement of water with hydrogen peroxide increases the level of sensitivity of the lanthanide ion causing an increased level of fluorescence when excited [12]. Lanthanide complexes are used due to their long lifetime, sharp emission spectrum, large stokes shift, absence of self-quenching, high quantum yield, and excellent solubility. In contrast to the majority of antioxidant assays, this fluorescent probe works without the need for enzymatic reactions or free radical generation, thus reducing complexity and cost.
Here, we test the hypothesis that the method developed by Durkop and Wolfbeis [12] could serve as a general measure of antioxidant activity, which is defined here as the reduction of hydrogen peroxide measured by the decrease in fluorescence of the EuTc compound. The europium tetracycline hydrogen peroxide reduction assay (EHRA) was found to be a low cost, high throughout antioxidant assay, providing a quantitative measure of the ability of various compounds to reduce H2O2. The utility of this assay to measure the antioxidant activity of food components was assessed using raw brewing materials in the form of hop extracts. In addition, as the EHRA was also found to be effective in different solvents, therefore it may well prove to have a breadth of applications across clinical and industrial research and testing.
Materials and methods
Reagents
Six antioxidant compounds were purchased from Sigma-Aldrich (Sydney, Australia): l-cysteine hydrochloride (cysteine), α-tocopherol (vitamin E), 4-hydroxy-3-methoxycinnamic acid (ferulic acid), l-ascorbic acid (vitamin C), gallic acid, and quercetin. Europium hexahydrate, tetracycline hydrochloride, 3-(N-morpholino)propanesulfonic acid (MOPS), perchloric acid (70 %), were also obtained from Sigma-Aldrich. Hydrogen peroxide (30 %) was purchased from UNIVAR (Sydney, Australia). All solutions and reagents were made using double-distilled water (resistance = 18.2 mΩ/cm2). MOPS buffer (26 mM) was adjusted to pH 6.95 using 70 % perchloric acid. The EuTc reagent was prepared by dissolving 2.33 mg EuCl.36H2O and 1 mg tetracycline hydrochloride in 10 mL of MOPS buffer (Durkop et al. 2005).
Antioxidants
The six antioxidant compounds were dissolved in 60 % methanol (quercetin and α-tocopherol) or water (l-cysteine, ferulic acid, l-ascorbic acid, and gallic acid) to a stock concentration of 30 mM prior to running the antioxidant assay based on the compounds solubility.
Extraction of antioxidants from hops
Cones and leaves from Pride of Ringwood hops (green, kiln or freeze dried) were acquired from Foster’s Group, Australia. Prior to extraction, dry weight percentage was determined following overnight drying in an oven, 80 °C. The cones and leaf material were finely chopped and crushed in a mortar to produce a coarse powder. Several different extracts were produced for analysis.
Acetic acetate buffer. 50 mM solutions of acetic acid and sodium acetate were mixed 41:9 (v/v, pH 4.0). Absolute ethanol (EtOH) was added to give a final concentration of 20 % (v/v). The ground hops (1 g) were extracted using 29 mL of acetic acetate buffer. The extract was autoclaved for 15 min at 115 °C, 15 psi within a tightly closed vessel. After cooling, the extract was centrifuged at 800×g for 5 min to remove debris. The supernatant was then centrifuged at 9,000×g and aliquots were stored at −20 °C for analysis. This extraction method closely resembles the kettle boiling of hops in the brewing process.
Sequential. The ground hops (0.5 g) were extracted in seven successive steps using round bottom flasks under a condenser, with constant stirring. First, water was used as the solvent, followed by 10, 20, 40, 60, 80, and 95 % (v/v) EtOH. Each step involved extraction for 1 h at either room temperature (i.e., COLD, 21 °C) or with heating (i.e., HOT, 100 °C). The extract was then centrifuged at 5,000×g for 5 min (10 °C) and the resulting supernatant from each extraction step was stored at −20 °C for future analysis. The solvent used in the next step was then added (30 mL) to the pellet and the process repeated.
Nonsequential. The ground hops (0.5 g) were extracted in 30 mL of 95 % (v/v) EtOH (i.e., P95 % EtOH). Round-bottom flasks were used under a condenser with constant stirring for 1 h under either COLD or HOT conditions. Upon completion, the supernatant for each sample was centrifuged at 5,000×g for 5 min (10 °C) then stored at −20 °C.
Frozen samples from each extraction step were thawed overnight at 4 °C and subsequently centrifuged at 10,000×g for 10 min (4 °C) immediately prior to use.
EHRA standard curve
H2O2 was diluted to yield a stock concentration range of 0–4 mM, and 25 μL of each were added to microtitre plate wells containing 140 μL of MOPS buffer, 60 μL of EuTc reagent, and 25 μL of assay diluent (i.e., in ethanol, methanol, acetic acetate buffer, water, or MOPS buffer). The resulting fluorescence was measured 20 min after addition of the oxidant solution. Linoleic acid hydroperoxide (LAOOH), cumene hydroperoxide (CHP), peroxynitrite, menadione, and diamide were also tested in this way to determine sensitivity of EuTc probe to various oxidants.
EHRA
Prior to testing, 60 μL of EuTc reagent was reacted with 25 μL of oxidant working solution for 20 min within a microtitre plate well containing 155 μL of MOPS buffer. This gives a final oxidant concentration of 200 μM, allowing an antioxidant activity range of 0–200 μM. Fluorescence was measured 20 min after addition of the oxidant solution. Extract or antioxidant compound (10 μL) was added and mixed on a microtitre plate shaker. Fluorescence was measured again after 20 min incubation. In order to determine the effects of extracts and antioxidants on the EuTc reagent, water was added instead of oxidant; this served as the assay blank to determine the affects of the antioxidant tested on the background fluorescence. Antioxidant activity was calculated as the amount of hydrogen peroxide that was reduced measured by a decrease in fluorescence on the EuTc probe.
All fluorescence measurements were acquired on a Cary eclipse fluorescence spectrophotometer with microplate adapter. EHRA was conducted in a 96-well plate with all samples run in triplicate. Fluorescence was excited at 400 nm and emission was measured at 616 nm. Band passes were set at 5 and 10 nm for excitation and emission slits, respectively. PMT voltage was set at 600–650 V. All incubations and measurements were carried out at room temperature (22 °C).
DPPH assay
The DPPH assay was carried out as previously described [2]. Briefly, a 125 μM DPPH solution was made in 100 % (v/v) methanol immediately prior to use (A492nm of 0.450–0.500). A calibration curve was generated using a 30 mM ascorbic acid stock solution diluted to a range of 0–200 μM in methanol. Fifty microliters of sample was added to 150 μL of 125 μM DPPH and mixed vigorously on a microtitre plate shaker in the dark for 10 min at room temperature (22 °C). The absorbance was read at a wavelength of 492 nm. Absorbance measurements were acquired on a Multiscan EX (Thermo Electron Corporation). Antioxidant activity was calculated by the following equation: 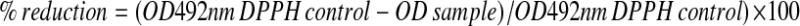 .
.
Results
Determination of EuTc probe sensitivity to various oxidants
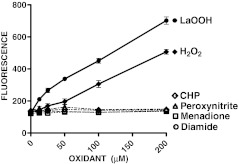
Several oxidants were incubated with the EuTc to determine whether different forms of oxidation would cause a change in fluorescence (Fig. 1). LAOOH and H2O2 caused an increase in fluorescence in relation to oxidant concentration. CHP, peroxynitrite, menadione, and diamide were shown to have no impact on the fluorescence of the EuTc probe over the concentration range tested (0–200 μM).
Fig. 1.
The effects of several oxidants on the fluorescence of the EuTc probe. Linoleic acid hydroperoxide (LAOOH), hydrogen peroxide (H2O2), cumene hydroperoxide (CHP), peroxynitrite, menadione and diamide (final concentration, 0–200 μM) were incubated with the EuTc probe and fluorescence was measured to determine level of sensitivity to oxidant (errors bars ±SD, n = 3)
Determination of antioxidant activity of various compounds
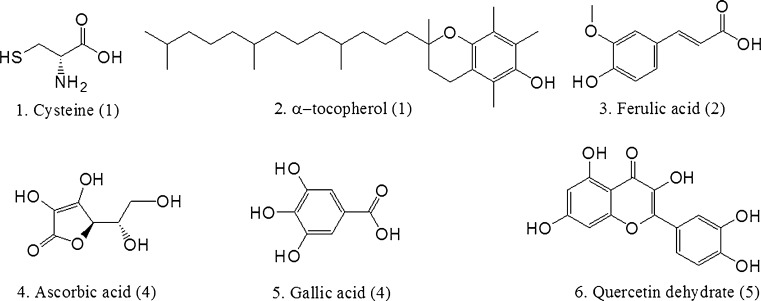
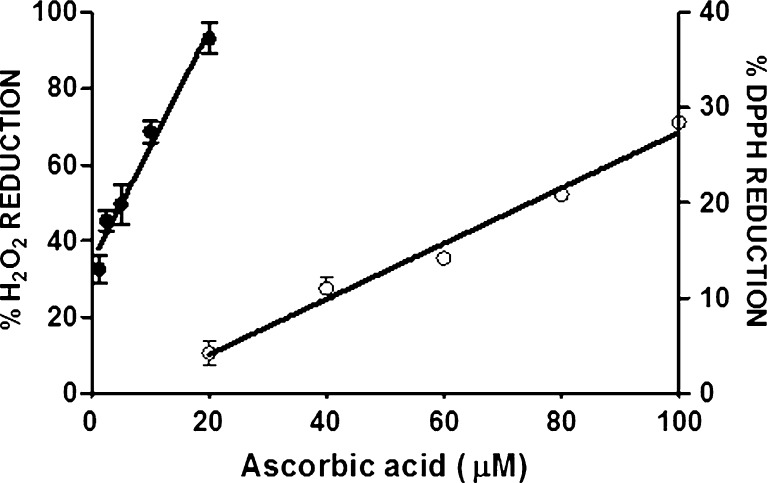
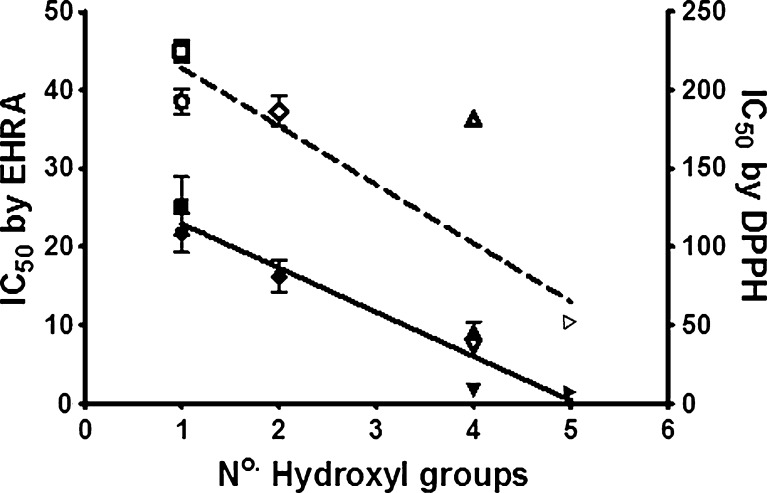
Several common antioxidant compounds with one to five hydroxyl groups (Fig. 2) were tested in parallel using the EHRA and the well-established DPPH assay; as an example, the calibration curves for ascorbic acid, generated by each of these assays, is shown (Fig. 3). The antioxidant compounds were chosen as they have been tested previously and shown to have measurable levels of antioxidant activity. The resulting IC50 values from the standard curves indicated a comparable trend in the two assays in terms of assessing antioxidant capacity (Table 1). Notably, the EHRA was substantially more sensitive than the DPPH assay (i.e., requiring 5- to 50-fold less antioxidant to establish an IC50), and had a larger dynamic range. In addition, the EHRA was highly sensitive to the number of hydroxyl groups in a given antioxidant molecule; there was a strong correlation between the IC50 of an antioxidant and the number of hydroxyl groups (R2 = 0.92 for the EHRA vs. 0.65 for the DPPH assay; Fig. 4).
Fig. 2.
The chemical structures of antioxidant compounds used in this study. The bracketed numbers represent the number of hydroxyl groups on each molecule
Fig. 3.
Standard curves generated using ascorbic acid for both the EHRA (R2 = 0.97, closed symbols) and DPPH assay (R2 = 0.98, open symbols). Errors bars ±SD, n = 3. Each point represents the percentage reduction of oxidant/radical after incubation
Table 1.
Antioxidant compounds comparing EHRA and DPPH reduction (errors shown are ±SD, n = 3)
| Antioxidant | EHRAa | DPPHa |
|---|---|---|
| Quercetin | 1.41 ± 0.45 | 51.86 ± 0.79 |
| Gallic acid | 1.68 ± 0.32 | 37.05 ± 0.84 |
| Ascorbic acid | 9.33 ± 1.06 | 181.64 ± 1.93 |
| Ferulic acid | 16.22 ± 2.01 | 186.64 ± 9.87 |
| Tocopherol | 21.74 ± 2.48 | 192.75 ± 8.33 |
| Cysteine | 25.20 ± 3.69 | 224.95 ± 6.73 |
aValues shown are the IC50 (micromole)
Fig. 4.
Antioxidant compounds tested by EHRA (R2 = 0.92, closed symbols) and DPPH assay (R2 = 0.65, open symbols). Each point represents the IC50 value obtained from the serial dilution of an antioxidant compound (errors bars ±SD, n = 3). Symbols represent 1 cysteine (square), 2 α-tocopherol (circle), 3 ferulic acid (diamond), 4 ascorbic acid (upward triangle), 5 gallic acid (downward triangle), 6 quercetin (right triangle)
Determination of antioxidant activity of hop extracts
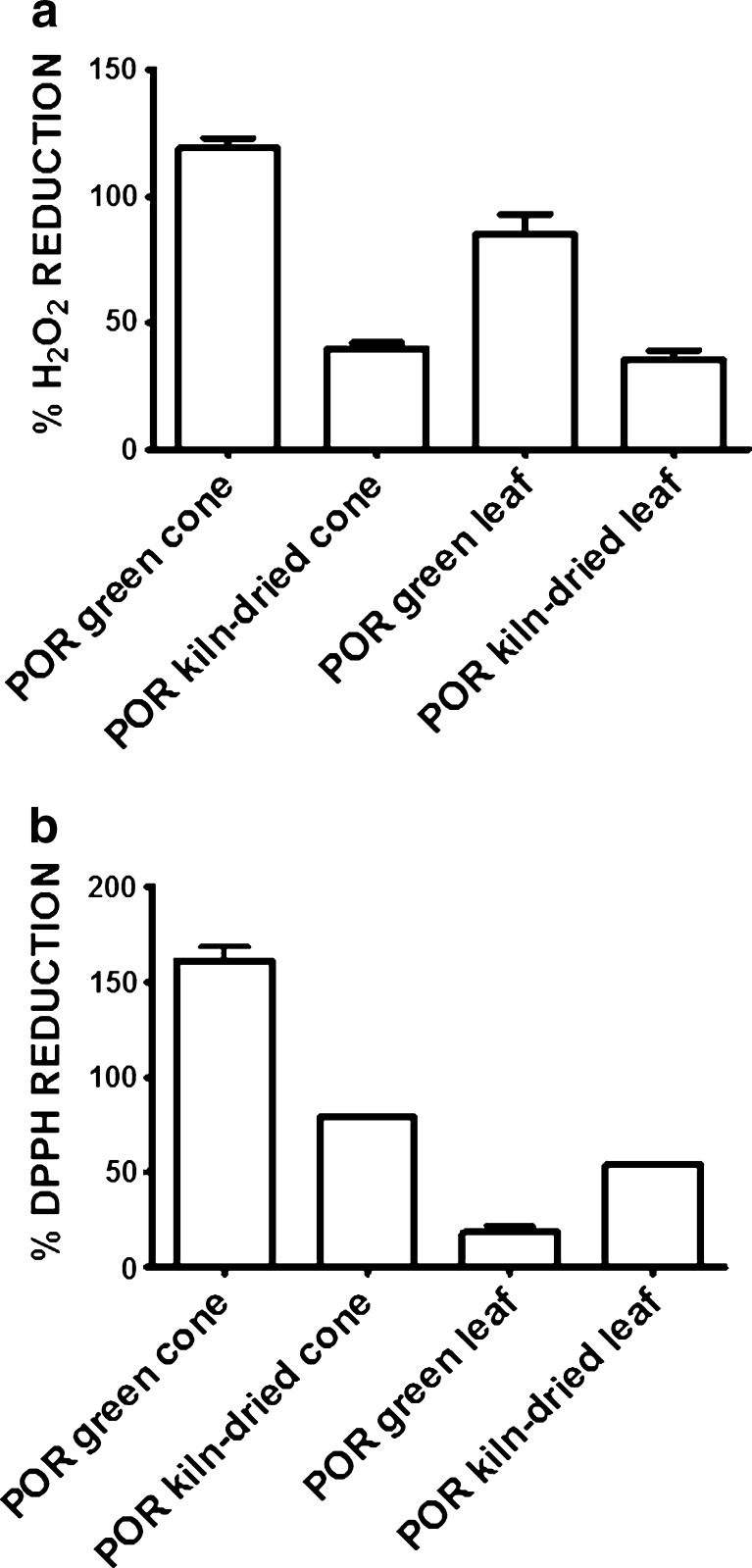
To test the applicability of the EHRA in the food and beverage industry, the antioxidant activity of hop extracts used in the production of lager beer was assessed. In the first instance, an acetic acetate buffer (pH 4.0) with 20 % EtOH (v/v) was chosen as it closely resembles the acidic environment of lager beer fermentation. Autoclaving at 115 °C was used to simulate kettle boiling as this process involves temperatures between 100 and 120 °C. Although the apparent antioxidant activity was always somewhat lower in the EHRA vs. the DPPH assay, both showed that green cone extracts had the highest level of antioxidant activity, and that kiln drying prior to extraction resulted in a 50 % or greater loss of antioxidant activity (Fig. 5). In contrast, the EHRA also identified a high level of antioxidant activity in the green leaf extract that was not detected by the DPPH assay (Fig. 5). Thus, the EHRA indicated that kiln drying consistently reduced the antioxidant activity in both cone and leaf extracts, a critical effect that was not reproducibly detected by the DPPH assay. According to the EHRA, the antioxidant activity of the green leaf extract was ∼70 % that of the green cone extract, and over 200 % higher than that of the kiln-dried cone extract, the latter being the most commonly used hop extract in lager beer fermentation.
Fig. 5.
Antioxidant activity of hop extracts assessed using the a EHRA and b DPPH assay. Errors bars ±SD, n = 3. Each point represents the percentage reduction of oxidant/radical after incubation
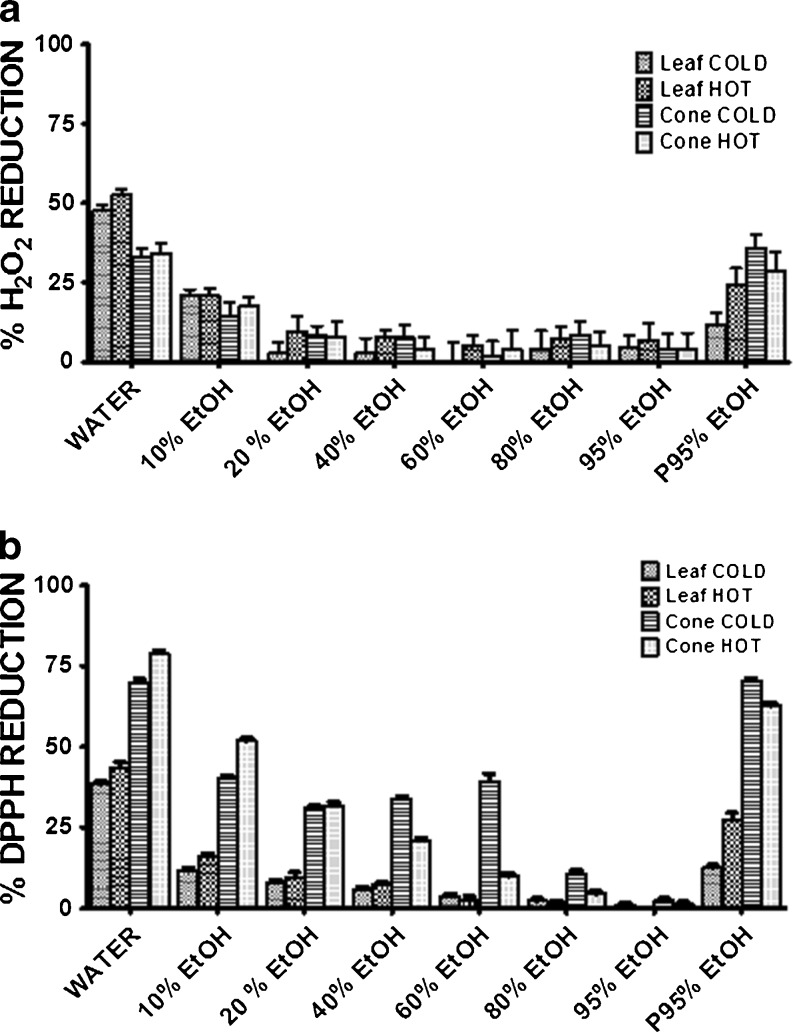
In an effort to more fully extract potential antioxidant compounds, further testing of hop extracts was carried out using both a sequential extraction process with increasing concentrations of EtOH, and a nonsequential process with 95 % EtOH (P95% EtOH). Kiln-dried extracts were chosen for this test as they are the most stable form of hop extract and are also used most frequently in the industry. Again, comparison of the EHRA and DPPH assay revealed similar trends but differing specific results (Fig. 6). Except for the P95% EtOH leaf extract, both assays showed that HOT extraction offered little or no advantages over COLD, and indeed, in the case of cone extracts, the DPPH assay indicated that heating was detrimental to antioxidant activity in some instances. In all cases, with the exception of the 80 and 95 % sequential EtOH extracts, the DPPH assay consistently identified a higher level of antioxidant activity in the cone extracts relative to those from leaf; except with water extraction alone, the EHRA indicated that cone and leaf extracts had comparable levels of antioxidant activity. Nonetheless, both assays identified water as consistently extracting the most antioxidant activity from both cones and leaves. Thus, in comparing the water and the P95% EtOH extracts, the results of both assays suggested that antioxidant compounds in the leaves are largely soluble, whereas the cones may contain compounds that are more readily extracted with the addition of EtOH.
Fig. 6.
Antioxidant activity in EtOH and water extracts from kiln-dried hops assessed using the a EHRA, and b DPPH assay (errors bars ±SD, n = 3). Each point represents the percentage reduction of oxidant/radical after incubation
Finally, three solvents (methanol, ethanol, and acetic acetate buffer) were tested with EuTc to determine whether high concentrations of solvent would alter the fluorescence of the complex. Very little change in fluorescence levels were observed with these solvents. Notably, however, the variance in the data was lowest in organic solvents (Table 2). As a whole, the data indicated that both hydrophilic and lipophilic antioxidants could be tested in parallel in future application.
Table 2.
Comparing the use of various solvents with the EHRA (errors represents ±SD, n = 3)
| Solvent | Fluorescence |
|---|---|
| Methanol | 140.71 ± 3.02 |
| Ethanol | 135.66 ± 1.93 |
| Acetic acetate buffer (pH 4.0) | 129.38 ± 5.61 |
| Water | 126.34 ± 7.66 |
| MOPS buffer (pH 6.95) | 126.33 ± 4.37 |
Discussion
The approach tested here enables the accurate determination of antioxidant activity in terms of hydrogen peroxide scavenging. The EHRA is highly sensitive, showing a strong correlation between antioxidant activity and the structure of the antioxidant molecule. We propose that the EHRA can be used to breach the hydrophilic/lipophilic gap in antioxidant screening as the assay works with many solvents. We, and others, also show that the EuTc probe is sensitive to a limited number of oxidants including hydrogen peroxide, linoleic acid hydrogen peroxide, and urea hydrogen peroxide [7]. Also, it is important to note that unlike many conventional antioxidant assays, the reagents for the EHRA, with the exception of the oxidant, can be made in advance. In an initial test for breadth of applicability, particularly in terms of potential applications in industrial testing, the EHRA has also identified a difference in antioxidant activity between extracts from hop cones and leaves.
The EHRA is thus both a robust and inexpensive assay to determine antioxidant activity and its design allows for a clear determination of antioxidant activity due to its pre-incubation of the EuTc probe with hydrogen peroxide. Addition of sample after this pre-incubation forces the antioxidant/s to compete with the EuTc probe for hydrogen peroxide; as hydrogen peroxide is reduced, water molecules bind to Eu3+ resulting in a decline in the level of fluorescence of the sensitized lanthanide ion. This also aids in limiting the level of interference from crude samples (i.e., containing high concentrations of ions or transition metals), as the EuTc probe is close to saturation with hydrogen peroxide. Several controls can be added to further validate the potential of the EHRA. In this report, we have used a measurement of activity based on background interference due to the incubation of EuTc probe with antioxidant/extract (i.e., without pre-incubation with hydrogen peroxide) and the linear activity of hydrogen peroxide reduction over a range of antioxidant/extract concentrations. It may be prudent to further test the effects of ions or transition metals on the fluorescence of the EuTc probe after saturation with hydrogen peroxide in order to measure the reduction in sensitivity. Overall, the EHRA is fast, simple, performs at near-neutral pH, is minimally sensitive to the presence of organic solvents, is not temperature dependent, and does not use enzymes or radical generators.
In some ways, the EHRA can be compared to the commercially available Amplex® Red assay kits. The Amplex® Red reagent works by reacting with hydrogen peroxide to yield Resorufin, a highly fluorescent compound. In order for the Amplex® Red assay to work, there must be at least one enzyme present, horse radish peroxidase, to catalyze the hydrogen peroxide/Amplex® Red reaction [49]. While the Amplex® Red reagent has been used for many applications including glucose, cholesterol, uric acid, and phosphate determinations, the compound exhibits a large spectral overlap for excitation and emission [49]. Also, as enzymes are necessary, the price per assay is approximately 50 times more expensive than the EHRA.
Common antioxidant assays used in the food and beverage industry are the DPPH assay and the oxygen radical absorbance assay (ORAC) and derivatives [10, 42]. The DPPH assay is cheap, simple, and uses a standard spectrometer to measure a decrease in absorbance; although widely used, the assay has some limitations in that it is not competitive and DPPH is both the probe and the source of oxidation [37]. Here, the DPPH assay appeared to indicate higher apparent levels of antioxidant activity than did the EHRA; however, this is due to the different method of oxidation between the two assays; the EHRA uses hydrogen peroxide and the DPPH assay uses free radical production for antioxidant measurement. These comparisons are thus relative rather than absolute. The ORAC assay was developed to determine the antioxidant activity of compounds found in serum, and was later optimized to measure brewing materials (i.e., wort, malt, and hops) [5, 27, 35]. This ORAC assay has proven to be useful but has the added complexity of being temperature sensitive and greatly affected by the solvent system used for oxidation. The pattern of results is similar for the EHRA and a derivative of the ORAC assay, which measures the inhibition of oxidation of linoleic acid by antioxidants [27]. That is, both assays determine a correlation antioxidant activity in relation to the number of oxidant molecules trapped per molecule of antioxidant.
It is well established that hops play a crucial role in protecting beer from spoilage through antioxidant activity [31, 32]. Many factors have been shown to contribute to the amount of antioxidant activity found in hops, including cultivar, season, soil composition, and extraction process [3, 6, 28, 36]. It has also been noted that hop leaves contain some degree of antioxidant activity and that this activity is not necessarily related to the activity found in the hop cones [38]. We have shown using the EHRA and DPPH assay that the assessed antioxidant activity of the hop extracts is based on the extraction solvent, temperature, and whether or not the material is dried. The green leaf and green cone material tested using the EHRA, with extraction methods closely related to brewing conditions, showed large amounts of antioxidant activity compared to the extracts from kiln-dried material. This has been shown previously using the DPPH assay, although a reduction of approximately 5 % was seen after kiln drying [24], in contrast to the EHRA which showed ∼70 % reduction. In other food components, heating/drying has also been shown to have a significant effect, decreasing antioxidant activity in tea leaf and coffee bean extracts by as much as 10 % using the DPPH assay or prevention of linoleic acid oxidation [8, 41]. When using water as the solvent on kiln-dried extracts, the EHRA showed that leaf extracts contain a higher level of antioxidant activity than the conventional hop cones used in industry. This was not detected by the DPPH assay, most likely due to the specificity of leaf extract activity towards H2O2 or to a difference in solubility of active compounds between leaves and cones. Both the DPPH assay and the EHRA showed similar results for COLD and HOT extracts of kiln-dried hops, pointing to the potential use of hop extracts in preventing oxidative damage to lager beer prior to the high temperatures of kettle boiling [26].
Hops have also been shown to be beneficial to health and have gained appeal due to the high level of “active” polyphenols such as resveratrol which protect against cancer, cardiovascular disease, and neurodegenerative disorders [1, 16–18]. Often, the link between level of protection, concentration of polyphenols, and antioxidant activity is strong, allowing for tests such as the EHRA to evaluate the potential of natural extracts to promote health [29]. The use of leaf material in the brewing industry could thus be a significant step forward as today it is primarily seen as a waste product.
To date, there has been no standardized method to test antioxidant activity. The International Congress on Antioxidant Methods was developed in 2004 for the purpose of setting such standards, yet resulted in division among scientists worldwide. It has been stated that one-dimensional methods for testing natural antioxidants are irrelevant and will not produce meaningful data [14]. For this reason, many authors agree that several methods of testing should be used to evaluate the complexity of antioxidant activity [21, 34, 37]. Several “ideal” requirements for antioxidant assays have been proposed in order to develop standardized methods for application [37]. Briefly, the need for a clear source of oxidation, a defined end-point and chemical mechanism, adaptability for both hydrophilic and lipophilic antioxidants, and ability to show reproducibility with high throughput. The EHRA is able to meet all these requirements and with minor adjustments can be made to work under various conditions (i.e., oxidant, solvent, temperature and pH). In combination with other methods, such as the well-established ORAC assay, which test the various mechanisms of antioxidant action, including metal chelation, free radical chain breaking, and oxygen scavenging, a wider understanding of the antioxidant–oxidant network can be established.
We thus propose that the EHRA can serve as a sensitive, robust, high throughput, and inexpensive adjunct to the arsenal of available antioxidant assays, equally at home in basic research as well as industrial labs and applications. This method allows for accurate determination of hydrogen peroxide reduction by natural extracts, here demonstrated for those important to the brewing process, which may help to promote the use of particular hop varieties and extraction processes.
Acknowledgements
The authors thank the University of Western Sydney and Carlton & United Breweries for support.
Glossary
- EHRA
Europium tetracycline hydrogen peroxide reduction assay
- EuTc
Europium tetracycline
- ROS
Reactive oxygen species
References
- 1.Bamforth CW. Nutritional aspects of beer—a review. Nut. Research. 2002;22:227–237. doi: 10.1016/S0271-5317(01)00360-8. [DOI] [Google Scholar]
- 2.Blios MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 3.Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing: science and practice. Cambridge: Woodhead; 2004. [Google Scholar]
- 4.Cano A, Hernandez-Ruiz J, Garcia-Canovas F, Acosta M. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem Anal. 1998;9:196–202. doi: 10.1002/(SICI)1099-1565(199807/08)9:4<196::AID-PCA395>3.0.CO;2-W. [DOI] [Google Scholar]
- 5.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Rad Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-R. [DOI] [PubMed] [Google Scholar]
- 6.Ceh B, Kac M, Kosir IL, Abram V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int J Mol Sci. 2007;8:989–1000. doi: 10.3390/i8090989. [DOI] [Google Scholar]
- 7.Courrol LC, Bellini MH, Tarelho LVG, Silva FRO, Mansano RD, Gomes L, Vieira ND, Jr, Shor N. Urea hydrogen peroxide determination in whole blood using europium tetracycline probe. Anal Biochem. 2006;355:140–144. doi: 10.1016/j.ab.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Daglia M, Papetti A, Gregotti C, Berte F, Gazzani G. In vitro and ex vivo protective activities of green and roasted coffee. J Agric Food Chem. 2000;48(5):1449–1454. doi: 10.1021/jf990510g. [DOI] [PubMed] [Google Scholar]
- 9.Diplock AT, Charleux J-L, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Vina-Ribes J. Functional food science and defence against reactive oxygen species. Brit J Nut. 1998;80(1):77–112. doi: 10.1079/BJN19980106. [DOI] [PubMed] [Google Scholar]
- 10.Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon J. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J Agric Food Chem. 2009;857:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 11.Duerkop A, Turel M, Lobnik A, Wolfbeis OS. Microtiter plate assay for phosphate using a europium-tetracycline complex as a sensitive luminescent probe. Anal Chim Acta. 2006;555:292–298. doi: 10.1016/j.aca.2005.09.007. [DOI] [Google Scholar]
- 12.Durkop A, Wolfbeis OS. Nonenzymatic direct assay of hydrogen peroxide at neutral pH using the EuTc fluorescent probe. J Fluor. 2005;15(5):755–761. doi: 10.1007/s10895-005-2984-6. [DOI] [PubMed] [Google Scholar]
- 13.Finley JW, Kong A, Hintze KJ, Jeffery EH, Ji L, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem. 2011;59:6837–6846. doi: 10.1021/jf2013875. [DOI] [PubMed] [Google Scholar]
- 14.Frankel EN, Meyer AS. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric. 2000;80:1925–1941. doi: 10.1002/1097-0010(200010)80:13<1925::AID-JSFA714>3.0.CO;2-4. [DOI] [Google Scholar]
- 15.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 16.Gasowski B, Leontowicz M, Leontowicz H, Latrich E, Lojek A, Ciz M, Tracktenberg S, Gorinstein S. The influence of beer with different antioxidant potential on plasma lipids, plasma antioxidant capacity, and bile excretion of rats fed cholesterol-containing and cholesterol-free diets. J Nutr Biochem. 2004;15:527–533. doi: 10.1016/j.jnutbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Gerhauser C. Beer constituents as potential cancer chemopreventative agents. Europ J Cancer. 2005;41:1941–1954. doi: 10.1016/j.ejca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Ghiselli A, Natella F, Guidi A, Montanari L, Fantozzi P, Scaccini C. Beer increases plasma antioxidant capacity in humans. J Nutr Biochem. 2000;11:76–80. doi: 10.1016/S0955-2863(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 19.Gutteridge JMC. Free-radical, damage to lipids, amino acids, carbohydrates and nucleic acids determined by thiobarbituric acid reactivity. Int J Biochem. 1982;14(7):649–653. doi: 10.1016/0020-711X(82)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JMC. Free radical, lipid peroxidation, and cell damage. J Biochem. 1984;219(1):1–14. [Google Scholar]
- 21.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 22.Kaneda H, Kano Y, Osawa T, Ramarathnam N, Kawakishi S, Kamada K. Detection of free radicals in beer oxidation. J Food Sci. 1988;53(3):885–888. doi: 10.1111/j.1365-2621.1988.tb08978.x. [DOI] [Google Scholar]
- 23.Kocherginsky NM, Kostetski YY, Smirnov AI. Antioxidant pool in beer and kinetics of EPR spin trapping. J Agric Food Chem. 2005;53:6870–6876. doi: 10.1021/jf051045s. [DOI] [PubMed] [Google Scholar]
- 24.Krofta K, Mikyska A, Haskova D. Antioxidant characteristics of hop and hop products. J. Inst. Brew. 2008;114(2):160–166. doi: 10.1002/j.2050-0416.2008.tb00321.x. [DOI] [Google Scholar]
- 25.Kuchel L, Brody AL, Wicker L. Oxygen and its reactions in beer. Packag Technol Sci. 2006;19:25–32. doi: 10.1002/pts.705. [DOI] [Google Scholar]
- 26.Lermusieau G, Liegeois C, Collin S. Reducing power of hop cultivars and beer ageing. Food Chem. 2001;72:413–418. doi: 10.1016/S0308-8146(00)00247-8. [DOI] [Google Scholar]
- 27.Liegeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hop against the 2,2′-azino-bis(2-amidinopropane) didryochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem. 2000;48:1129–1134. doi: 10.1021/jf9911242. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Gu X, Tang J, Liu K. Antioxidant activities of hop (Humulus lupulus) and their products. J Am Soc Brew Chem. 2007;65(2):116–121. [Google Scholar]
- 29.Lugasi A. Polyphenol content and antioxidant properties of beer. Acta Alimentaria. 2003;32(2):181–192. doi: 10.1556/AAlim.32.2003.2.7. [DOI] [Google Scholar]
- 30.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243(21):5753–5760. [PubMed] [Google Scholar]
- 31.Mikyska A, Hrabak M, Haskova D, Srogl J. The role of malt and hop polyphenols in beer quality, flavour and haze stability. J Inst Brew. 2002;108(1):78–85. doi: 10.1002/j.2050-0416.2002.tb00128.x. [DOI] [Google Scholar]
- 32.Mikyska A, Krofta K, Haskova D, Culik J, Cejka P. The Influence of hopping on formation of carbonyl compounds during storage of beer. J Inst Brew . 2011;117(1):47–54. doi: 10.1002/j.2050-0416.2011.tb00442.x. [DOI] [Google Scholar]
- 33.Miller NJ, Diplock AT, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 34.Moon J, Shibamoto T. Antioxidant assays for plant and food components. J Agric Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 35.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 36.Priest MA, Boersma JA, Bronczyk SA. Effects of aging on hop and liquid CO2 hop extracts. Am Soc Brew Chem. 1991;49:98–101. [Google Scholar]
- 37.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 38.Psenakova I, Hetesova L, Nemecek P, Farago J, Kraic J. Genotype and seasonal variation in antioxidant activity of hop extracts. Agric (Pol’mohospodarstvo) 2010;56:106–113. [Google Scholar]
- 39.Quick KL, Hardt JI, Dugan LL. Rapid microplate assay for superoxide scavenging efficiency. J Neurosci Methods. 2000;97:138–144. doi: 10.1016/S0165-0270(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 40.Rakicioglu Y, Perrin JH, Schulman SG. Increased luminescence of the tetracyline–europium(III) system following oxidation by hydrogen peroxide. J Pharm Biomed Anal. 1999;20:397–399. doi: 10.1016/S0731-7085(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 41.Satoh E, Tohyama N, Nishimura M. Comparison of the antioxidant activity of roasted tea with green, oolong and black teas. Int J Food Sci Nutr. 2005;56(8):551–559. doi: 10.1080/09637480500398835. [DOI] [PubMed] [Google Scholar]
- 42.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson WH, Biawa JP, Miracle RE, Bamforth CW. Laboratory scale studies of the impact of oxygen on mashing. J Inst Brew. 2003;109(3):273–283. doi: 10.1002/j.2050-0416.2003.tb00168.x. [DOI] [Google Scholar]
- 44.Uchida M, Ono M. Determination of hydrogen peroxide in beer and its role in beer oxidation. J Am Soc Brew Chem. 1999;57(4):145–150. [Google Scholar]
- 45.Vanderhaegen B, Neven H, Verachtert H, Derdelinckx G. The chemistry of beer ageing—a critical review. Food Chem. 2006;95:357–381. doi: 10.1016/j.foodchem.2005.01.006. [DOI] [Google Scholar]
- 46.Whitehead TP, Thorpe GH, Maxwell SRJ. Enhanced chemiluminescence assay for antioxidant capacity in biological fluids. Anal Chim Acta. 1992;266:265–277. doi: 10.1016/0003-2670(92)85052-8. [DOI] [Google Scholar]
- 47.Wolfbeis OS, Schaferling M, Durkop A. Reversible optical sensor membrane for hydrogen peroxide using an immobilized fluorescent probe, and its application to a glucose biosensor. Micochim Acta. 2003;143:221–227. doi: 10.1007/s00604-003-0090-5. [DOI] [Google Scholar]
- 48.Wu M, Lin Z, Wolbeis OS. Determination of the activity of catalase using a europium(III)–tetracycline-derived fluorescent substrate. Anal Biochem. 2003;320:129–135. doi: 10.1016/S0003-2697(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 50.Zufall C, Tyrell T. The influence of heavy metal ions on beer flavour stability. J Inst Brew. 2008;114(2):132–142. doi: 10.1002/j.2050-0416.2008.tb00318.x. [DOI] [Google Scholar]