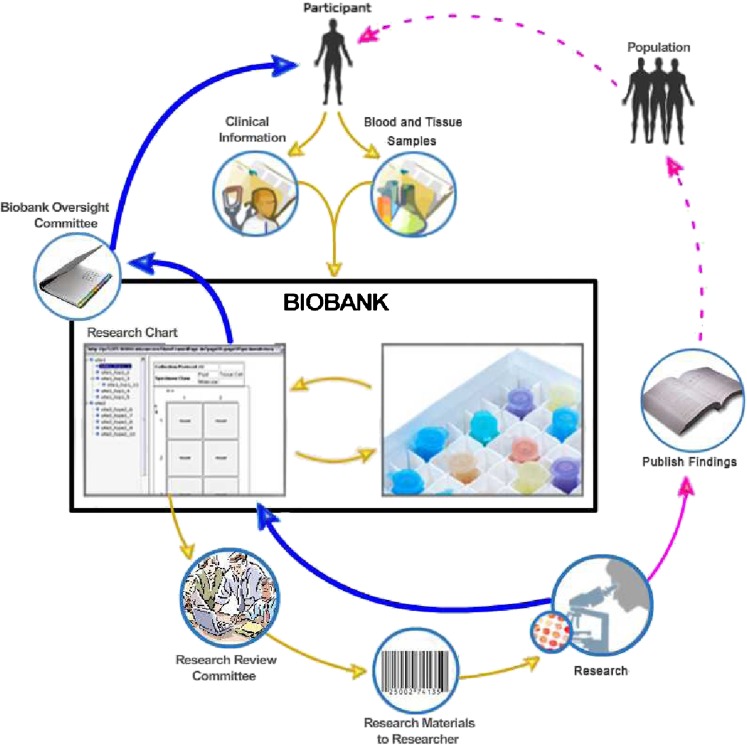
Fig. 1.
Lifecycle of a Biobank (modified from the original image which is in the public domain that can be found at: https://cabig.nci.nih.gov/tools/catissuesuite). This figure depicts the enrollment of a participant into a biobank through the reporting and return of results. Yellow arrows represent the movement of samples and data from the participant into the biobank and the movement of de-identified samples and data from the biobank to researchers. Pink arrows represent the movement of research results from the researchers to the public, while dashed lines represent the possible dissemination of benefits. Blue arrows represent the movement of research results back into the biobank and back to the participant. In the biobank figure, two governance junctures are portrayed: the research review committee (RRC) and the biobank oversight committee (BOC). The RRC is designed to evaluate whether a researcher’s proposal meets ethical and scientific standards and then permits the distribution of de-identified samples. The RRC may or may not be a formal institutional review board (IRB) since de-identified samples are non-human subjects and do not require IRB oversight. The BOC is designed to evaluate when and whether to return research results to individual participants. Some biobanks are designed not to permit the return of results, and in such biobanks this committee would not exist. Currently, there are no legal requirements as to the BOC’s membership or the criteria it would use to decide when to return results