Figure 7. dpA3 forms temperature-dependent secondary structure.
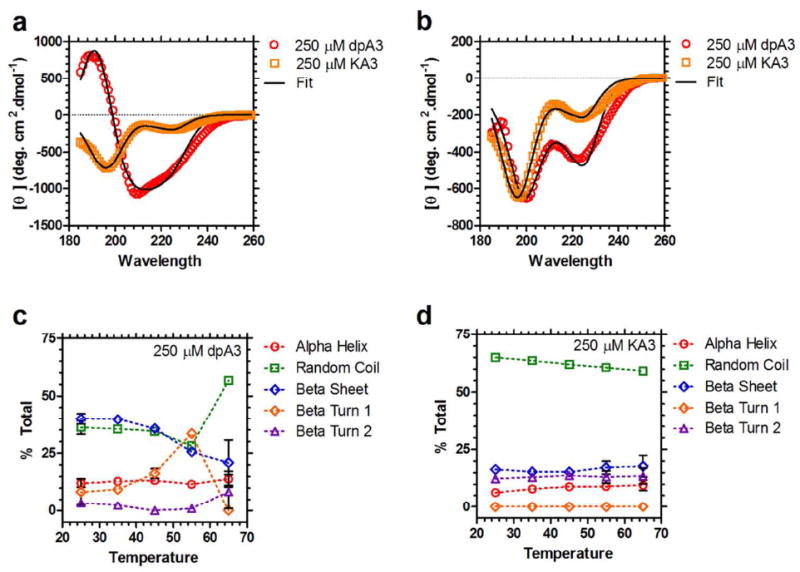
(a) Circular dichroism (CD) spectra for dpA3 (250 μM, red) and KA3 (250 μM, orange) below the transition temperature (T = 55 °C). The spectrum for the unmodified KA3 (R2=0.9930) peptide is considerably different from the dipalmitoylated dpA3 (R2=0.9922), suggesting that nanofiber assembly induces formation of partial secondary structure. (b) Above the transition temperature for dpA3 (T = 65 °C) there is a noticeable change in the molar ellipticities (red, R2=0.9832); however, the unmodified peptide KA3 (orange, R2=0.9913) remains unchanged. (c) At lower temperatures the majority of the dpA3 (250 μM) population adopts either a β-sheet or random coil conformation. On heating there is an increase in the β-turn 1 component suggesting that β-turn 1 formation is necessary for the phase separation of this class of ELPAs. (d) The major conformation of unmodified KA3 at all temperatures is random coil. There is no significant change in the secondary structure at higher temperatures.
