Figure 8.
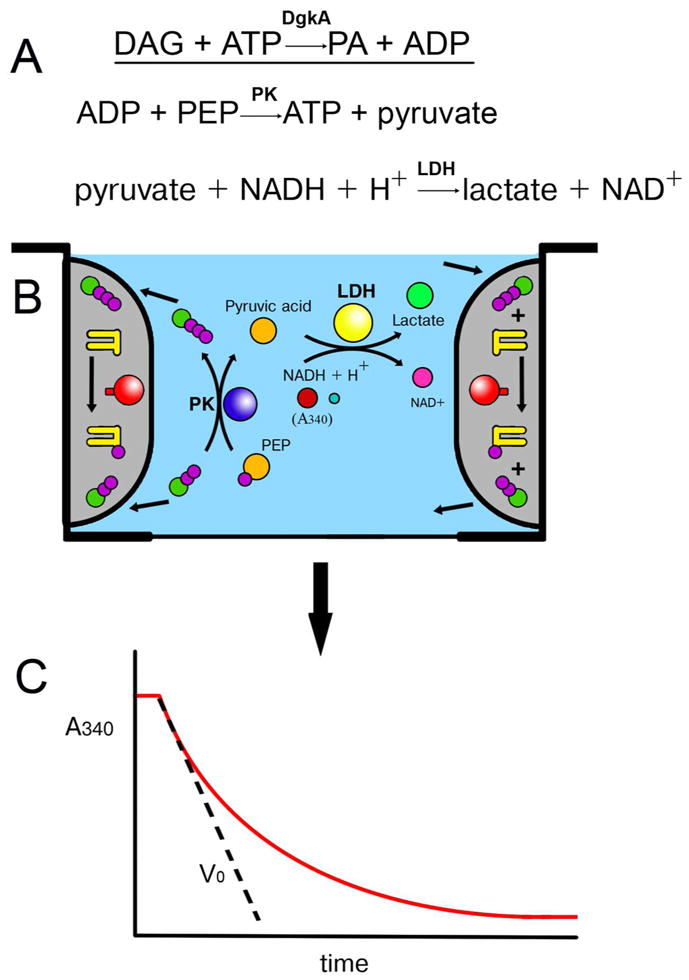
Monitoring the γ-phosphoryl group transfer activity of diacylglycerol kinase (DgkA) reconstituted into the bilayer of the cubic phase by a coupled enzyme assay method (A) in a muli-well plate.62 Protein-laden mesophase (shaded grey in (B)) is positioned on the wall of the well where it remains in place throughout the assay as a consequence of its intrinsic viscosity and stickiness. The mesophase is bathed in buffer (blue) containing the water-soluble ingredients of the coupled assay (ATP, ADP, pyruvate, lactate, phosphoenolpyruvate (PEP), NADH/NAD+, pyruvate kinase (PK) and lactate dehydrogenase (LDH)). Water soluble substrate, ATP, diffuses into the nanoporous mesophase for use by DgkA in synthesizing phosphatidic acid from diacylglycerol both of which reside in and are confined to the bilayer of the mesophase. Water soluble product, ADP, diffuses out of the mesophase into the bathing solution where it is used by the coupled enzyme assay system to regenerate ATP. The coupling process that involves PK and LDH leads to a drop in the concentration of NADH which, in turn, is monitored continuously in situ in a multi-plate reader by a reduction in absorbance at 340 nm of the bathing solution (C). The slope of the progress curve (C) provides a measure of the initial velocity, Vo, as indicated.
