Figure 1.
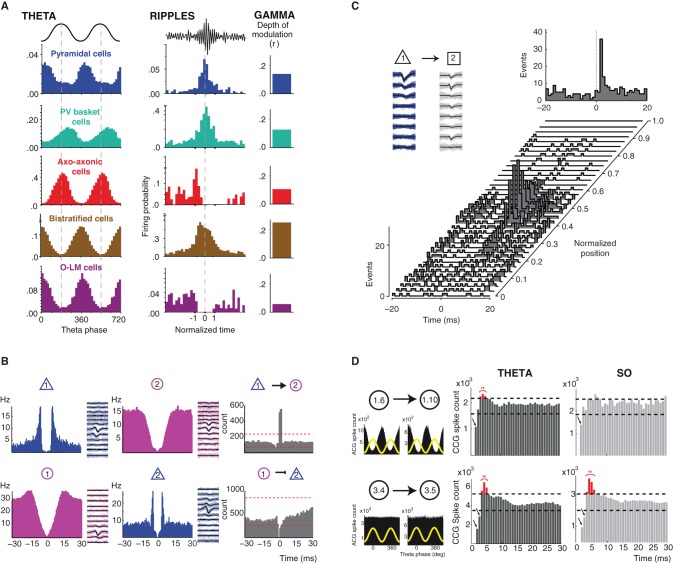
Brain state/oscillation dependent modulation of neuronal activity and functional connectivity. (A) Oscillation-dependent firing profiles. Distinct classes of hippocampal GABA neurons display different and specific firing patterns (firing probability histograms) during theta and ripple oscillations (their spike timing is coupled to field gamma oscillations to differing degrees). Modified from (Klausberger and Somogyi, 2008). (B) Identification of putative functional connectivity among neurons. Autocorrelograms and average filtered waveforms of a putative principal cell (blue) and an interneuron (purple) in the entorhinal cortex in layer 2 (top panel) and layer 3 (bottom panel). Cross-correlogram (CCG, grey) reveals short-latency monosynaptic excitation between neuron 1 and neuron 2 (top panel) and short-latency suppression of spikes in the target principal neuron (bottom panel). Modified from (Quilichini et al., 2010). (C) Behavior-dependent changes in monosynaptic interactions. Short-term cross-correlograms between a putative pyramidal cell (cell 1, mean waveform in black and single spikes in blue) and interneuron (cell 2, mean waveform in black and single spikes in grey) in the medial prefrontal cortex as a function of the rat's position on the central arm of a figure-eight-T-maze before a left turn. A significant functional connection between the cell 1 and 2 is only revealed by the CCGs around the center of the arm. Top right panel, cross-correlograms session mean. Modified from (Fujisawa et al., 2008). (D) Modulation of functional connectivity by brain state dependent oscillations. In the entorhinal cortex superficial layers (2 and 3), a portion of pairs between putative interneurons (1.6 presynaptic neuron; 1.10 postsynaptic neuron) displaying a strong theta-phase modulation of their firing (top left panel, theta phase distribution of spikes in black and theta cycle as yellow wave) show a brain state dependent expression of post-inhibitory rebound (PIR) only during theta oscillations (red bins in the CCGs). However, the expression of PIR did not depend upon the oscillatory activity (theta vs. slow oscillations) in theta-phase unmodulated pairs of putative interneurons (3.4 presynaptic neuron; 3.5 postsynaptic neuron; bottom panel). Modified from (Adhikari et al., 2012).