Figure 3.
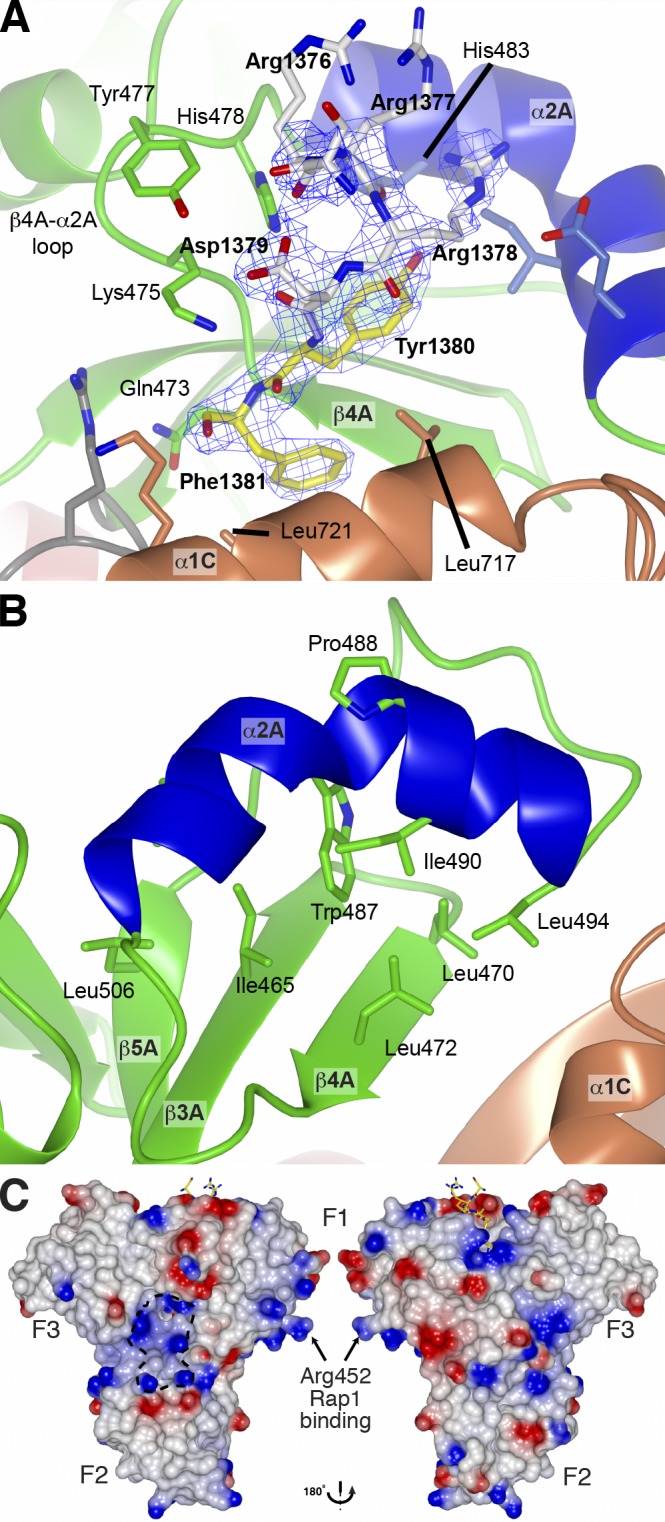
Structural details of the HEG1 binding pocket in KRIT1. (A) Electron density of the HEG1 peptide in complex with the KRIT1 FERM domain. Electron density (2 Fo-Fc map contoured at 1.2 σ) of the HEG1 peptide. The HEG1 peptide is colored as in Fig. 2 C, and the KRIT1 FERM domain is as in Fig. 2 B. Some of the key KRIT1 residues are highlighted. (B) Close view of the novel helix α2A in the KRIT1 F1 domain. The helix is kinked by ∼70° in the middle because of the presence of Pro488. The helix position is stabilized by hydrophobic contacts with residues from the β sheet. (C) The surface charge map of the KRIT1 FERM domain shows a basic surface at the F1–F2–F3 subdomain interface. The position of Arg452 is highlighted as mutation of this residue to Glu reduces KRIT1 binding to Rap1.
