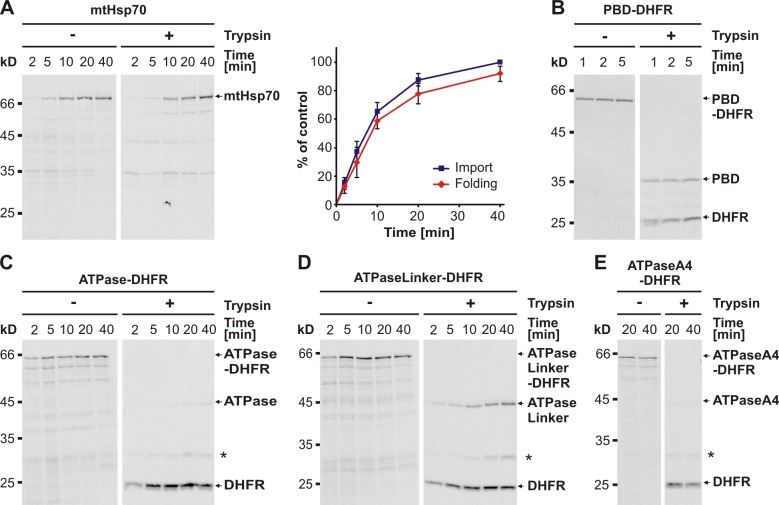
Figure 1.
The chaperone mtHsp70 and its individual domains fold rapidly in organello. (A–E) Folding of mtHsp70 (A), PBD-DHFR (B), ATPase-DHFR (C), ATPaseLinker-DHFR (D), and ATPaseA4-DHFR (E) upon import into mitochondria. Radiolabeled precursor proteins were imported into wild-type mitochondria for the time periods indicated. Nonimported material was digested with trypsin. Mitochondria were reisolated and solubilized. Half of the sample was mock treated (−) to assess import, and the other half was treated with trypsin (+) to assess the folding state of the imported mtHsp70. Samples were analyzed by SDS-PAGE and autoradiography and, in the case of mtHsp70, quantification by densitometry (A, right). The amount of mtHsp70 imported at the latest time point was set to 100%. The graph represents the mean values ± standard deviation (n = 5). The asterisks indicate the folded DHFR domain with nondigested spacer.