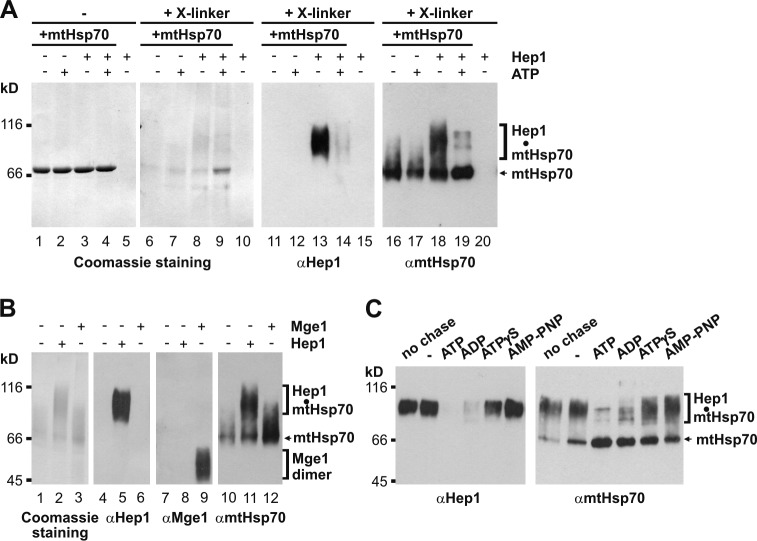
Figure 6.
A folding intermediate of mtHsp70 interacts with Hep1. (A and B) Unfolded mtHsp70 was diluted into the buffer containing Hep1 in twofold molar excess over mtHsp70 in the presence or absence of ATP, as indicated (A and B), or in a twofold molar excess of Mge1 (B). Samples were incubated at 30°C for 20 min and then treated with or without the cross-linking reagent (glutaraldehyde). Samples were analyzed by SDS-PAGE and Coomassie staining. Aliquots of the samples (15%) were analyzed by SDS-PAGE and immunodecoration with antibodies against Hep1, mtHsp70 (A and B), and Mge1 (B). (C) Unfolded mtHsp70 was incubated with a twofold molar excess of Hep1 for 10 min at 30°C (no chase). Samples were further incubated for 10 min in the absence (−) or presence of nucleotides, when indicated. All samples were treated with glutaraldehyde and analyzed as described in A.