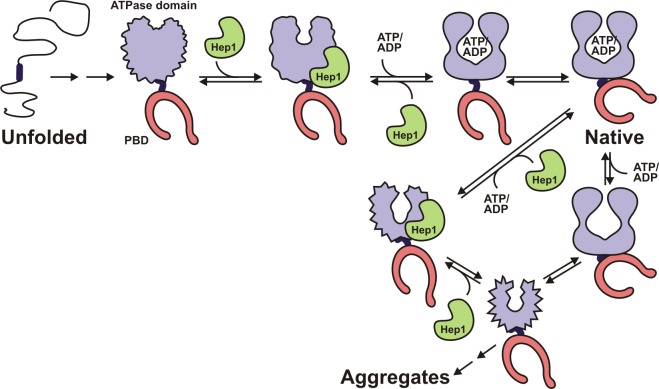
Figure 7.
Model of the folding of mtHsp70. The ATPase domain together with the interdomain linker and the PBD fold as individual units in the de novo folding pathway of mtHsp70. The PBD folds on its own; the ATPase domain does not. The ATPase domain in the context of the linker adopts a folding intermediate that is recognized by Hep1. Binding of Hep1 induces conformational changes that allow binding of adenine nucleotides (ATP/ADP). Hep1 is released. The folded ATPase domain and the PBD together form the native and catalytically active mtHsp70. Because the PBD folds independently of any factor, it is presented in a folded state in all folding intermediates, although PBD and ATPase domain probably fold in parallel. The model also depicts the transition of native mtHsp70 in its nucleotide-free state into an aggregation-prone conformer. Hep1 binds to the conformer, preventing aggregation and allowing mtHsp70 to regain its native structure.