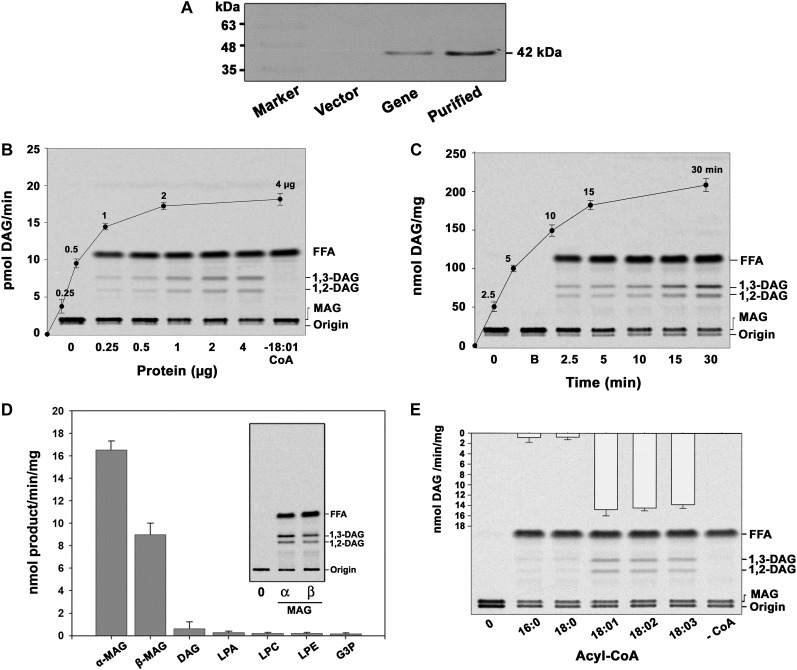
Figure 2.
The isolated cDNA clone encodes MGAT. AhMGAT and its corresponding vector were transformed into wild-type yeast. A, Yeast cells overexpressing AhMGAT was confirmed by western-blot analysis using anti-His monoclonal antibody. B, Recombinant AhMGAT was purified by Ni2+-NTA column chromatography, and the dialyzed protein was used as the enzyme source. MGAT activity was determined with increasing amounts of protein (0–4 µg) for 15 min with [14C]MAG and 20 µm oleoyl-CoA. Lane 0, Enzyme was added after stopping the reaction. FFA, Free fatty acid. C, The time-dependent acylation of MAG; the reaction was initiated by the addition of 2 µg of protein. The reaction was stopped by extracting lipids, and the lipids were separated on a silica-TLC plate using petroleum ether:diethyl ether:acetic acid (70:30:1, v/v) as the solvent system. Lane 0, Enzyme was added after stopping the reaction; lane B, enzyme fraction was boiled for 5 min, and assay was performed (boiled enzyme control). D, The preference for various acyl acceptors was determined using 2 µg of protein, 50 μm acyl acceptor, and 20 μm [14C]oleoyl-CoA. The inset represents a typical phosphor image of MGAT assay with [14C]oleoyl-CoA and α,β-MAG as substrates. Lane 0, Enzyme was added after stopping the reaction. G3P, Glycerol-3-phosphate; LPE, lysophosphatidylethanolamine. Values are means ± sd of three independent experiments. E, Preference of acyl-CoAs. The assay was performed with 50 μm [14C]MAG with 20 μm of different acyl-CoAs. Lane 0, Enzyme was added after stopping the reaction. Values are means ± sd of three independent determinations.