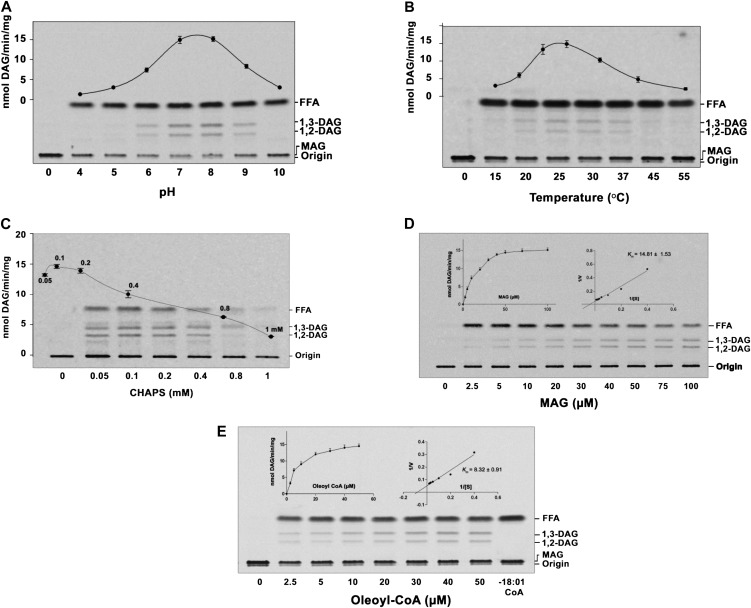
Figure 3.
Characterization of AhMGAT. The enzyme activity was performed under different assay conditions with the purified AhMGAT protein for 10 min. A, MGAT assay was performed at different pH values with 50 µm [14C]MAG (prepared with CHAPS) and 20 µm oleoyl-CoA at 30°C. FFA, Free fatty acid. B, Temperature dependence of MGAT activity. C, MGAT activity was monitored in the presence of various concentrations of CHAPS (0.05–1 mm). Values are means ± sd of three independent experiments. D, Lineweaver-Burk plot of AhMGAT toward MAG. Activity was measured as a function of MAG concentration, and 20 µm [14C]oleoyl-CoA was kept constant. Values are averages of two independent determinations. E, Lineweaver-Burk plot of AhMGAT toward oleoyl-CoA. Activity was measured with 50 µm [14C]MAG and increasing concentrations of oleoyl-CoA. Lane 0, Enzyme was added after stopping the reaction. Values are averages of two independent determinations.