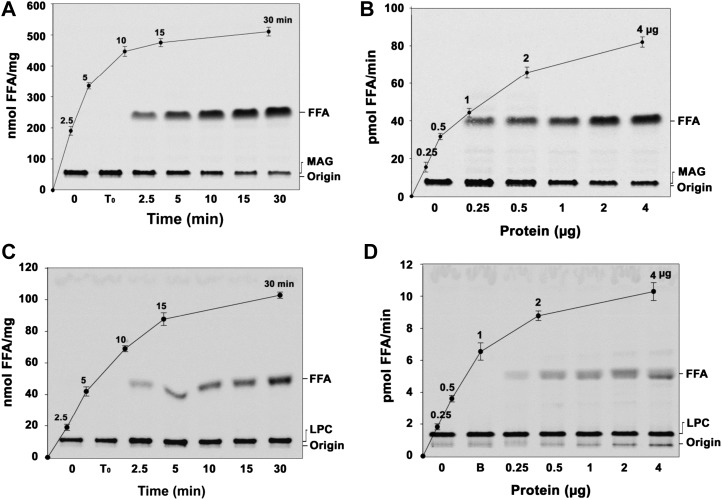
Figure 4.
The peanut MGAT exhibits both MAG and LPC hydrolase activities. A, Time-dependent hydrolysis of MAG at 30°C with 2 µg of recombinant protein. Lane 0, Enzyme was added after stopping the reaction; lane T0, zero time point (reaction was stopped immediately after adding the enzyme). FFA, Free fatty acid. B, Protein-dependent MAG hydrolase assay was conducted with 50 µm [14C]MAG for 10 min at 30°C. Lane 0, Enzyme was added after stopping the reaction. The reaction was stopped by extracting lipids, and the lipids were separated on a silica-TLC plate using petroleum ether:diethyl ether:acetic acid (70:30:1, v/v) as the solvent system. Values are means ± sd of three independent experiments. C, The time-dependent LPC hydrolase assay was performed using 50 µm [14C]LPC with 2 µg of recombinant AhMGAT. Lane 0, Enzyme was added after stopping the reaction; lane T0, zero time point (reaction was stopped immediately after adding the enzyme). D, The LPC hydrolase assay was performed for 10 min at 30°C with increasing amounts of purified AhMGAT. Lane 0, Enzyme was added after stopping the reaction; lane B, enzyme fraction was boiled for 5 min, and assay was performed. The reaction was stopped and lipids were resolved on a TLC plate using chloroform:methanol:28% ammonia (65:25:5, v/v) as the solvent system. Values are means ± sd of three independent experiments.