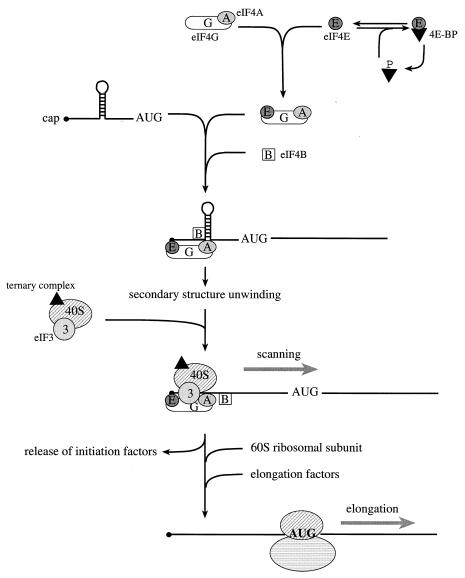
Figure 3.
The initiation and elongation phases of translation in eukaryotes. In starved or stressed cells, the cap binding protein eIF4E is sequestered by hypophosphorylated 4E-BPs. In growing or stimulated cells, the 4E-BPs are hyperphosphorylated to release eIF4E, such that it can interact with the scaffolding protein, eIF4G. In conjunction with the RNA helicase eIF4A and the cofactor eIF4B, 5′ secondary structure is melted, and a small ribosomal subunit is recruited to a single-stranded, cap-proximal region of an mRNA via an interaction between eIF4G and the ribosome-associated factor eIF3. The small ribosomal subunit, along with a ternary complex composed of eIF2, GTP, and Met-tRNAi, then scans the mRNA in a 5′ to 3′ direction until an AUG start codon in the proper sequence context is encountered. At this point, initiation factors are released, and the large ribosomal subunit is recruited. The elongation factors catalyze aminoacyl-tRNA binding to ribosomes, and the translocation of the mRNA from the ribosomal A site to the P site.