Abstract
Serotonin (5HT) is a pronociceptive mediator in the periphery and evidence implicates involvement in trigeminal pain processing. However, the mechanism(s) by which 5HT modulates trigeminal nociceptors remains unclear. Trigeminal pain can be evoked by the transient receptor potential V1 channel (TRPV1), which is expressed by nociceptive trigeminal neurons and induces release of proinflammatory calcitonin gene-related peptide (CGRP). In our preclinical models, 5HT evoked thermal hyperalgesia and enhanced calcium influx and CGRP release from the TRPV1 population of trigeminal nociceptors. Whether this occurs in humans is unknown. As dental pulp is densely innervated by trigeminal nociceptors, routine tooth extractions offer a unique opportunity to examine whether 5HT enhances CGRP release from human nociceptors. Pulpal tissue was collected from 140 extracted molar teeth from men and women and basal release samples were collected prior to treatment with saline or 5HT 100 μM. CGRP release was then stimulated with the TRPV1 agonist capsaicin 1 μM and quantitated by enzyme immunoassay. Additional samples were collected for western blots to examine 5HT receptor expression. We report that 5HT induced a significant increase in capsaicin-evoked CGRP release and this enhancement was observed only in female dental pulp, with no effect of 5HT on male dental pulp. The greatest amount of CGRP release occurred in dental pulp from women in the luteal phase of the menstrual cycle. These results indicate that 5HT enhances capsaicin-evoked CGRP release from human trigeminal nociceptors in a sexually dimorphic manner providing a mechanistic basis for prevalence of trigeminal pain disorders in women.
Keywords: CGRP, 5HT, craniofacial, orofacial, teeth, pain
1. Introduction
As approximately one in four people experience persistent craniofacial pain mediated by the trigeminal system, such as toothache and temporomandibular joint disorder pain [31–32] and approximately 10 million Americans experience migraine [41], craniofacial pain represents a prevalent and problematic burden, especially to the higher proportion of patients suffering from improperly controlled pain [6; 19]. Interestingly, trigeminal pain is more prevalent in women compared to men. For instance, migraine is twice as prevalent in women [30–31; 43] and varies across the menstrual cycle [42; 47]. However, it is unclear whether this sex difference in prevalence is due to biological differences, psychosocial differences, environmental differences, or to multiple factors.
The monoamine neurotransmitter serotonin (5HT) has been implicated in trigeminal pain, including migraine and masseter muscle pain [13–14; 20; 23] and 5HT is known to evoke hyperalgesia when injected into human tissues [4; 13–14]. Therapeutics targeting the 5HT system have proven successful in treating some forms of trigeminal pain [2; 10; 16; 39]. However, it remains unclear how 5HT modulates human trigeminal nociceptors. Preclinical animal models have provided insight into the mechanisms by which the trigeminal system mediates pain and indicate that 5HT may regulate a subpopulation of trigeminal sensory neurons that express the transient receptor potential (TRPV1) ion channel, which is critical in transducing many noxious stimuli. TRPV1 is gated by thermal stimuli [7–8; 12; 46], oxidized linoleic acid metabolites [35–36; 38] and inflammatory mediators [9; 37] to induce calcium influx in nociceptors resulting in release of inflammatory peptides, such as calcitonin gene-related peptide (CGRP). There is evidence in dorsal root ganglia sensory neurons that 5HT potentiates TRPV1 functions [24; 34; 40] and prolongs nociceptor excitation during inflammation [1; 22]. We recently reported that 5HT evokes calcium influx in capsaicin-sensitive rat trigeminal sensory neurons with a corresponding enhancement of CGRP release [27] and that 5HT2A and 5HT3A receptor antagonists and the anti-migraine drug sumatriptan (5HT1B/1D agonist), attenuate 5HT enhancement of capsaicin-evoked CGRP release in vitro and thermal hyperalgesia in vivo [26]. However, due to limitations of readily available human tissues, translation of these mechanistic findings into clinical relevance has been challenging.
Human dental pulp is composed of many of the same cells, fibers and nociceptors as other human tissues, thus offering a readily available model for the study of human nociceptors [21]. Nociceptors innervating human dental pulp express TRPV1 and contain the nociceptive neuropeptide CGRP [15]. Using a recently developed in vitro superfusion method to measure CGRP release from human dental pulp [15], we tested our hypothesis that 5HT enhances capsaicin-evoked CGRP release from human nociceptors. We also performed western blotting in human dental pulp to analyze the expression of 5HT receptors known to be involved in peripheral pain processing (5HT1B, 5HT1D, 5HT2A and 5HT3A) in aims of providing insight into 5HT’s ability to modulate human nociceptors.
2. Materials and Methods
2.1. Subjects
Fifty-five patients (22 males and 33 non-pregnant, post-pubertal, pre-menopausal females) between the ages of 14 and 40 presenting to the clinics of the University of Texas Health Science Center at San Antonio School of Dentistry consented to participate in this study. A total of 140 teeth were collected from enrolled patients who had already elected to have one to four third molars (“wisdom teeth”) extracted. Selected teeth were limited to a clinical diagnosis of a normal pulp lacking caries or restorations with no periapical radiolucencies upon radiographic evaluation and normal responsiveness to sensory testing. All teeth used in this study showed fully developed roots to ensure that the innervation of the pulp was complete. All patients were anesthetized using nerve block injection consisting of 2% lidocaine with epinephrine, with 78% of patients also receiving intravenous fentanyl and midazolam (50–100 ug and 5 mg, respectively) during extractions. Patient data collected for analysis included gender, age, ethnicity (Table 1), current medications, hormone-releasing birth control usage and day since last menses. All studies were approved by the University of Texas Health Science Center at San Antonio Institutional Review Board and patients provided written informed consent to participate in the study.
Table 1.
Demographic Data of Dental Pulp Donors
| Gender | N donors | n teeth | Age in years mean (range) | Ethnicity |
|---|---|---|---|---|
| Male | 22 | 45 | 22 (15–31) | 23% Hispanic (N=5) |
| 55% White Non-Hispanic (N=12) | ||||
| 14% African-American (N=3) | ||||
| 4% Japanese/Asian (N=1) | ||||
| 4% Persian/Indian (N=1) | ||||
| Female | 33 | 95 | 22 (16–31) | 39% Hispanic (N=13) |
| 52% White Non-Hispanic (N=17) | ||||
| 9% Japanese/Asian (N=3) |
2.2 Materials
Capsaicin (TRPV1 agonist; Sigma, St. Louis, MO) was dissolved in ethanol stock and stored in aliquots at -20oC. A fresh aliquot was diluted for each experiment with final dilutions containing <0.5% ethanol. Serotonin hydrochloride (5HT; Sigma) was dissolved in double-distilled water and diluted immediately prior to each use. All experimental vehicles were made at the same time and of the same constitution as the experimental drug.
2.3. In vitro superfusion of human dental pulp
Within 30 minutes of extraction, teeth were bisected and coronal pulp tissue was removed and placed into wells containing Hanks buffered saline solution (pH 7.4; HBSS; Invitrogen, San Diego, CA). Following an in vitro superfusion technique previously described [15], capsaicin-responsiveness was determined by incubating the pulpal tissue (1 sample per well) in HBSS buffer alone for 20 min (basal sample) followed by a 20 min exposure to vehicle or capsaicin (1 μM, 10 μM or 30 μM; n=15–18 per group). To determine the effect of pretreatment with 5HT, pulpal tissue was incubated for 20 min in vehicle or 5HT (100 μM), a concentration shown to significantly enhance capsaicin-stimulated CGRP release in sensory neuron cultures [27], prior to stimulation with capsaicin (1 μM; n=32–35 per group). An additional group of 48 teeth from female patients were added to this group to conduct secondary analysis of the effects of hormone-releasing birth control use or menstrual cycle status on CGRP release. Upon completion of the superfusion experiments, tissue samples were lysed to release total cellular stores of CGRP by freezing/thawing the samples. Each superfusate sample (baseline, pretreatment and capsaicin stimulated) was analyzed for CGRP levels by human-specific CGRP enzyme-linked immunoassay (SPI-BIO, Montigny le Bretonneux, France). Data is expressed as % of basal release to account for individual pulp variability and avoid artificial inflation.
2.4. Western Blot
Protein was extracted from the dental pulp of 7 male and 6 female human third molar teeth. Teeth were cut on a sagittal plane and the pulp was extracted. The pulp was quickly placed in ice cold PBS then transferred into ice cold homogenization buffer. Tissue homogenization was performed at 50 oscillations/sec utilizing a Tissue Lyzer LT (Qiagen; Valencia, CA) and a 5 mM stainless steel bead. The pulp/homogenization buffer solution was lysed in 2 minute intervals, for a total of 4 minutes to maintain cool temperature of solution. Protein was then quantified utilizing a bicinchoninic acid (BCA) assay. Equal amounts of protein were loaded and separated by polyacrylamide gel electrophoresis on a 12% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad; Hercules, CA). The membrane was then blocked with 5% milk in TBS-Tween 20 and 5HT receptors were probed with selective antibodies for either goat anti-5HT1B receptor (1:500; 42 kDa; Santa Cruz; Santa Cruz, CA), goat anti-5HT1D receptor (1:500; 43 kDa; Santa Cruz), rabbit anti-5HT2A receptor (1:500; 53 kDa; Immunostar; Hudson, WI) or rabbit anti-5HT3A receptor (1:1000; 53 kDa; Proteintech, Chicago, IL) and monoclonal mouse anti-β-actin (1:1000; Santa Cruz) as control. Membranes were then treated with either donkey anti-goat (1:2500; Santa Cruz), goat anti-rabbit (1:2500 or 1:5000) or goat anti-mouse (1:5000; Santa Cruz) secondary antisera linked to horseradish peroxidase for signal amplification and detected with an enhanced chemiluminescence reagent kit (Amersham; Piscataway, NJ). To confirm antibody selectivity, blots were pre-incubated in blocking peptides for the 5HT1B (1:500; Santa Cruz), 5HT1D (1:500; Santa Cruz) and 5HT2A (1:500; Immunostar). A blocking peptide was unavailable for the 5HT3A antibody; a lack of staining was confirmed by omitting the primary antibody. Images were captured with an AlphaImager Gel Documentation system (Cell Biosciences; Santa Clara, CA) and brightness/contrast values were maximized for publication with Adobe Photoshop CS4.
2.5. Data analysis
Superfusion data are presented as mean ± SEM percentage of basal levels. Western blots were analyzed by densitometry using Image J-64 (http://rsb.info.nih.gov/ij/). Each blot was selected as a region of interest (ROI), sampled three times and analyzed for average gray scale pixel value (sum gray values/number of pixels; 16-bit) following background correction (set at ~150 pixels)[17]. Data are reported as percentage of β-actin controls. Statistical analyses using GraphPad Prism software version 5 (GraphPad, San Diego, CA) were conducted by unpaired t-test, or one-way or two-way analysis of variance (ANOVA) and post-hoc individual group analyses were compared using Bonferroni post hoc test. The statistical significance was tested at p<0.05. Error bars represent S.E.M.
3. Results
3.1. Capsaicin evokes significant, concentration-dependent CGRP release from human dental pulp
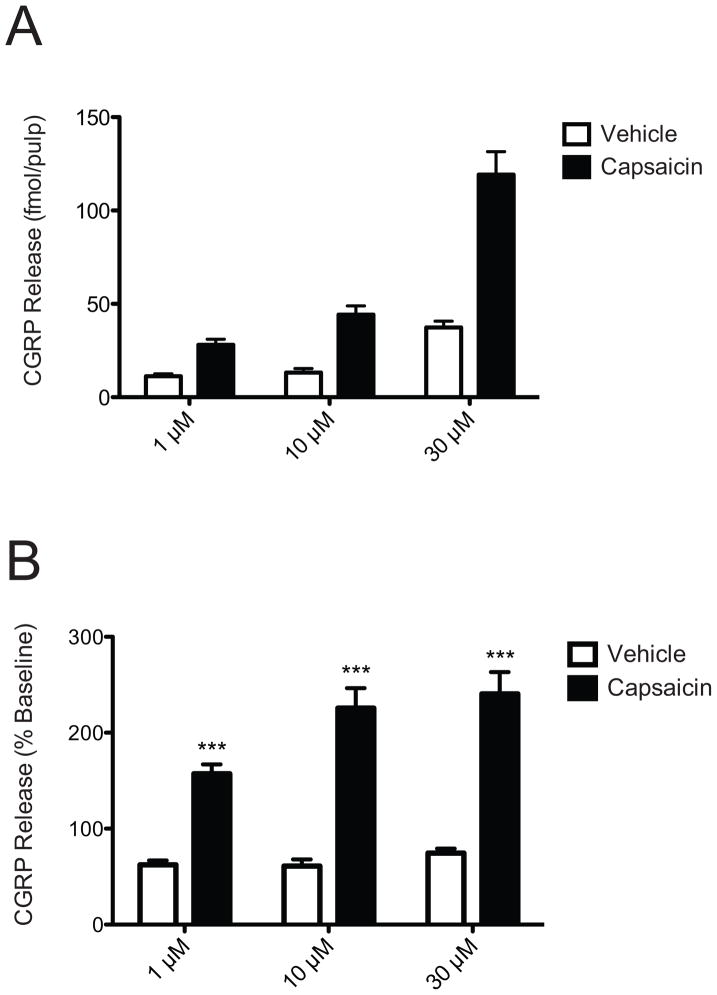
Capsaicin alone evoked a concentration-dependent increase in CGRP release ranging from 28 ± 3 to 119 ± 12 fmol/pulp at 1–30 μM compared to vehicle controls (Figure 1A). When analyzed as percent of basal CGRP release per pulp, this increase in CGRP release was significant across all concentrations compared to vehicle [F(2,92)=4.04; p<0.05], with the 10 and 30 μM concentration more than doubling CGRP release (Figure 1B). The 10 and 30 μM concentrations produced significantly greater degree of CGRP release compared to the 1 μM concentration. The 1 μM concentration was chosen for the next set of experiments to permit detection of a potential enhancement of capsaicin-evoked CGRP release.
Figure 1.
Capsaicin evokes significant CGRP release from human dental pulp. Capsaicin (1 – 30 μM; closed bars) induces CGRP release in a concentration-dependent manner from human dental pulp compared to vehicle (open bars) as expressed by amount fmol released per pulp (A). The same data is expressed as percentage of basal release (B). * indicates significance at p<0.05 compared to vehicle; n=15–18 teeth per group.
3.2. Serotonin enhances capsaicin-stimulated CGRP release from female but not male human dental pulp
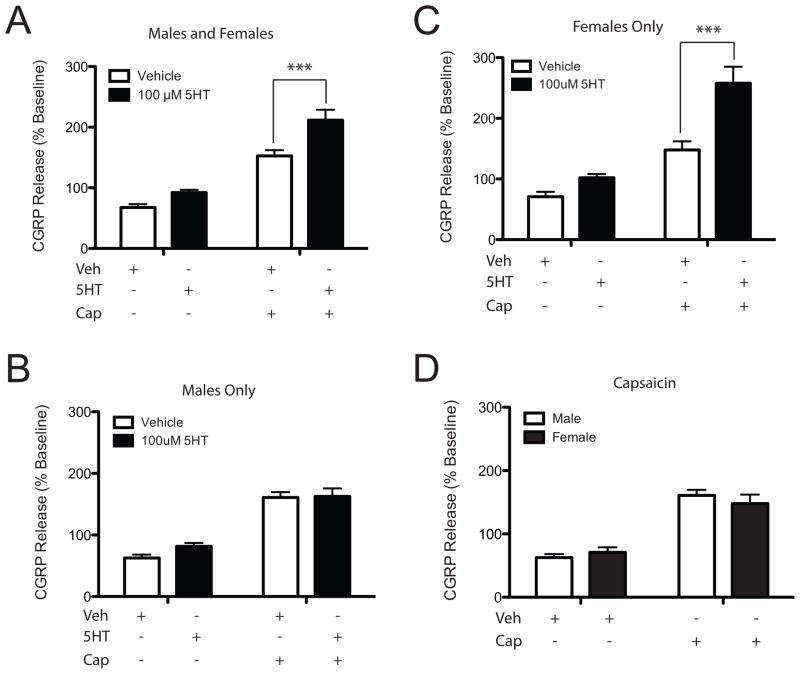
Pretreatment with 5HT 100 μM significantly increased capsaicin-stimulated CGRP release by ~50% [F(1,134)=14.88; p<0.05], while 5HT alone did not evoke CGRP release (p>0.05) (Figure 2A). When these data were stratified by sex, there was no significant effect of 5HT on capsaicin-stimulated CGRP release from male dental pulp [F(1,54)=1.18; n.s.] (Figure 2B). However, 5HT pretreatment evoked a significant, two-fold enhancement of CGRP release in dental pulp from females [F(1,72)=20.15; p<0.05] (Figure 2C). This effect was independent of whether the tooth was erupted or non-erupted prior to extraction [t(16)=1.53; n.s.]. The observed sexually dimorphic effect of 5HT-enhancement of capsaicin-evoked CGRP release in human dental pulp may be due to differences in CGRP concentrations or TRPV1 levels. To evaluate these possibilities we administered capsaicin alone to male and female dental pulp and measured evoked CGRP release. Capsaicin alone did not evoke a significantly different level of CGRP release from male and female dental pulp [F(1,60)=0.05; n.s.] (Figure 2D).
Figure 2.
5HT significantly enhances CGRP release in female, but not male, human dental pulp. 5HT (100 μM; closed bars; n=35) compared to vehicle (open bars; n=32) does not have an effect on CGRP release alone, but does significantly enhance capsaicin 1 μM -evoked CGRP release from tooth pulp (males and females combined; A). When stratified by sex, there is no effect of 5HT on capsaicin-evoked CGRP release from male dental pulp (n=17) compared to vehicle (n=12; B). 5HT (n=18) significantly enhanced capsaicin-evoked CGRP release from dental pulp of females compared to vehicle (n=20; C). Capsaicin or vehicle alone induced a comparable release of CGRP from the dental pulp of males (open bars, n=12) and females (closed bars; n=20; D). * indicates significance at p<0.05 compared to vehicle.
3.3. Serotonin receptors are expressed at similar levels in male and female human dental pulp
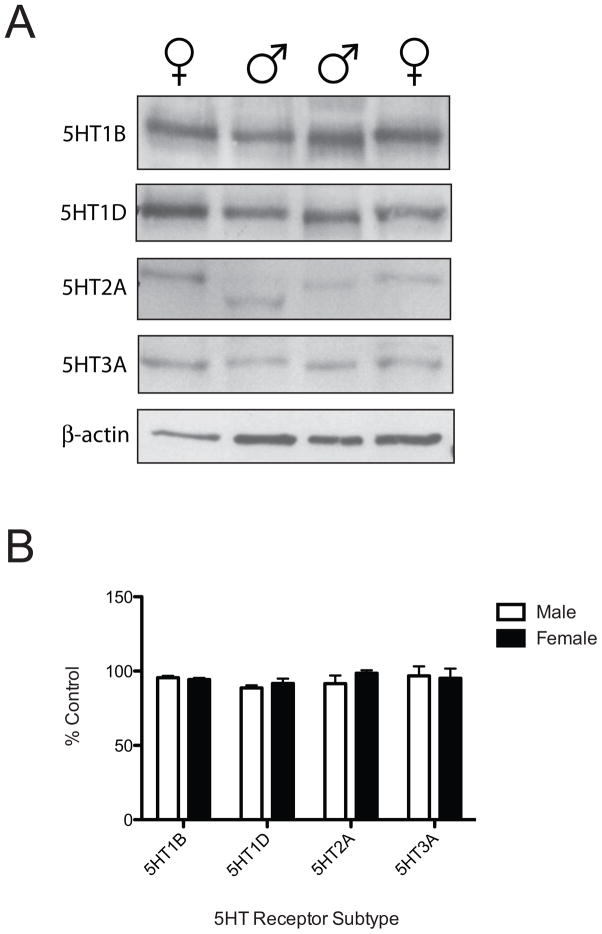
The expression of 5HT receptors in human dental pulp was examined by western blot to confirm the availability of anatomical targets for 5HT modulation. The 5HT1B, 5HT1D, 5HT2A and 5HT3A receptor protein was expressed in both male and female human dental pulp (Figure 3A). Densitometry on the western blots revealed no significant differences in the expression of these receptor subtypes between male and female dental pulp [t(11)=0.69, 0.86, 1.12, 0.18 respectively; n.s.] (Figure 3B).
Figure 3.
5HT receptor protein is expressed in human dental pulp and comparable between the sexes. Western blot bands illustrating 5HT1B, 5HT1D, 5HT2A and 5HT3A receptor expression in male versus female human dental pulp (A). Quantification of western blots indicates no sex differences in expression; n=7 male, n=6 female (B).
3.4. Effect of age and ethnicity on 5HT enhancement of capsaicin-stimulated CGRP release from male and female human dental pulp
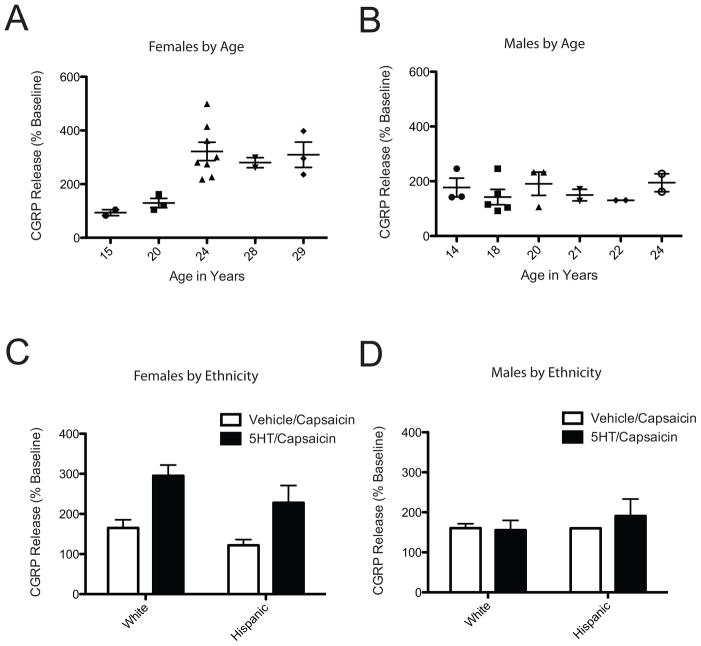
5HT evoked consistently higher capsaicin-evoked CGRP release from dental pulp of females over 24 years of age compared to 15–20 years of age [F(3,17)=5.76; p<0.05] (Figure 4A). There was no effect of age on capsaicin-stimulated CGRP release following vehicle pretreatment [F(5,19)=2.886; n.s.] and there was no significant effect of age on dental pulp from males [F(5,16)=0.58; n.s.] (Figure 4B). The vast majority of dental pulp was from non-hispanic white and hispanic patients. When stratified by these two groups, there was no significant effect of ethnicity on 5HT enhancement of CGRP release from female [F(1,34)=3.52; n.s.] (Figure 4C) or male dental pulp [F(1,16)=0.27; n.s.] (Figure 4D).
Figure 4.
There is a significant effect of age, but not ethnicity, on 5HT enhancement of CGRP release from female dental pulp. Effect of 5HT on capsaicin-evoked CGRP release stratified by age in females (A; n=18) verses males (B; n=17) and by ethnicity in females (C; n=8 white non-hispanic and 10 hispanic) verses males (D; n=10 white non-hispanic and 7 hispanic). * indicates significance at p<0.05 compared to vehicle.
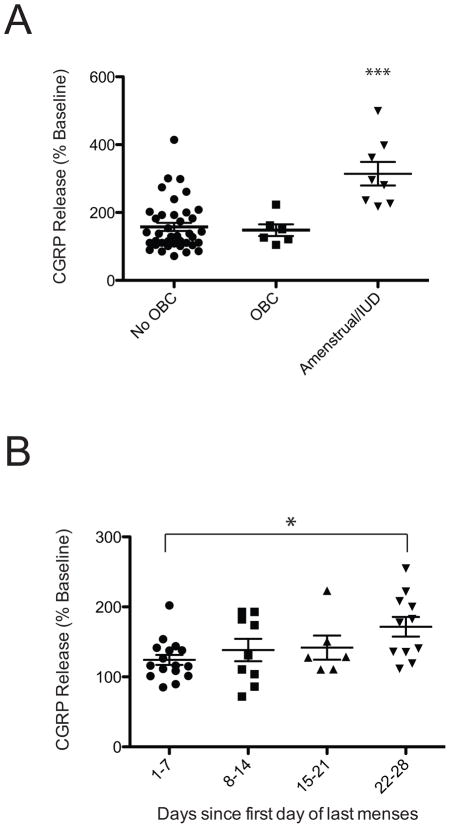
3.5. Female patients that were amenstrual due to hormonal IUD or in the week before menses presented with the greatest levels of 5HT-enhanced CGRP release
The CGRP release data from the dental pulp of female patients were divided into three groups based on their current use of hormonal manipulations to prevent pregnancy: use of oral birth control (OBC), no use of oral birth control (No OBC) or amenstrual due to the use of a synthetic progestogen-releasing interuterine device (Amenstrual/IUD). 5HT-enhanced CGRP release was significantly higher in dental pulp from females who were amenstrual due to IUD [F(2,52)=14.92; p<0.05] (Figure 5A). 5HT-enhanced CGRP release was similar in the dental pulp of females regardless of the use of oral birth control. The CGRP release data from the dental pulp of female patients not using hormonal manipulations (No OBC) was then further divided into four groups based on the first day of the last menses: 1–7, 8–14, 15–21, 22–28 days. There was a significant effect of the status of menstrual cycle on 5HT enhancement of capsaicin-stimulated CGRP release [F(3,41)=3.06; p<0.05] (Figure 5B). 5HT enhanced CGRP release was lowest in dental pulp from females in the week during menses (1–7), while 5HT-enhanced CGRP release was highest in dental pulp from females in the week prior to menses (22–28). In contrast, there was no significant effect of day since last menses on CGRP release evoked by capsaicin alone [F(3,18)=1.714; n.s.].
Figure 5.
5HT enhanced CGRP release to a greater degree in dental pulp from female patients during the week prior to menses. Effect of 5HT on capsaicin-evoked CGRP release grouped by teeth from females not on oral birth control (No OBC), on oral birth control (OBC) or amenstrual due to hormone-releasing IUD implantation (Amenstrual/IUD; A). Data from patients not on birth control is also expressed as days since first day of last menstrual cycle (B). * indicates significance at p<0.05 compared to vehicle.
4. Discussion
5HT is a pronociceptive mediator in the periphery that has been reported to enhance TRPV1-evoked CGRP release from rat trigeminal sensory neurons [27]; however, whether this occurs in human nociceptors is unknown. Using an in vitro superfusion assay on human dental pulp, here we report that (1) 5HT enhances capsaicin-evoked CGRP release from female dental pulp, but not male dental pulp, and (2) that 5HT-enhanced CGRP release varies across age and the menstrual cycle. Furthermore, we report that there are no significant sex differences in 5HT1B, 5HT1D, 5HT2A or 5HT3A receptor protein expression in human dental pulp and that capsaicin evokes similar levels of CGPR release from both male and female peptidergic terminals.
Capsaicin steadily evoked a concentration-dependent increase in CGRP release consistent with that previously reported [15]. CGRP release to vehicle treatment was consistently lower than basal levels likely due to further stabilization of the extracted dental pulp. Capsaicin produces maximal CGRP release at 60 μM without inducing detectable desensitization, which is observed at 100 and 300 μM in dental pulp [15]. In this study, capsaicin 1 – 30 μM was examined to allow for potential enhancement of capsaicin-evoked CGRP release without inducing desensitization. Similar to previous studies [15], there was no detectable effect of local nerve block in the absence or presence of intravenous fentanyl/midazolam on CGRP release from human dental pulp. As capsaicin 1 μM was the lowest concentration to evoke a significant increase in CGRP release when analyzed as percentage of basal release, this concentration was chosen to stimulate CGRP release following pretreatment with either 5HT or vehicle. When dental pulp was pretreated with 5HT prior to capsaicin, capsaicin-evoked CGRP release was significantly enhanced. Importantly, 5HT alone did not evoke a significant change in CGRP release. Together, these data indicate that 5HT modulates TRPV1-evoked CGRP release in human dental pulp. This modulation may be occurring via direct or indirect actions on TRPV1-expressing nociceptors in human dental pulp. 5HT receptors may be located on non-neuronal cells; however, it is unlikely that 5HT modulates CGRP release through non-TRPV1 expressing cells as 5HT given alone did not alter CGRP release.
As trigeminal pain is more prevalent in women, the present data were then stratified by sex of the dental pulp donor to determine potential sex differences in 5HT-evoked CGRP release. Indeed, 5HT pretreatment enhanced capsaicin-stimulated CGRP release from the dental pulp of females only. There was no significant effect of 5HT pretreatment on the dental pulp from males. It is important to note that capsaicin evoked comparable CGRP release from male and female dental pulp, indicating that the observed effect is due to differences in serotonergic sensitization of TRPV1 neurons. As there was no sex differences in capsaicin-evoked or 5HT-evoked CGRP release nor was there a difference in the 5HT receptor proteins analyzed, a remaining possibility is that 5HT receptor coexpression on TRPV1 fibers is sexually dimorphic. As there are current limitations on available specific 5HT receptor antibodies, this possibility remains untested. There was also no effect of whether the donors were of non-hispanic white or hispanic ethnicity on CGRP release. Together, these data provide evidence of a sexually dimorphic biological mechanism that may contribute to the well recognized sex differences in some forms of trigeminal pain. While it has been reported that 5HT-evoked rat trigeminal afferent discharge is not sexually dimorphic [45], our data indicate that 5HT may instead alter pain transmission indirectly via enhancing TRPV1-evoked CGRP release.
It is possible that the observed sexually dimorphic serotonin effect is mediated by the actions of sex steroids. Estradiol potentiates capsaicin-mediated currents in rat dorsal root ganglia sensory neurons [11] and female rodents exhibit greater capsaicin-evoked nocifensive responses during proestrous [28], when estrogen levels peak. To examine whether 5HT-enhanced CGRP release is altered across the menstrual cycle, we stratified our data first by whether the donor was on oral or IUD-mediated birth control. Interestingly, dental pulp from patients who were amenstrual due to a hormone-releasing IUD [29] represented the highest levels of 5HT-enhanced CGRP release. The data from females on oral birth control and approximately half of the females not on oral birth control were similar to CGRP release observed in male pulp (~170%), while the data from amenstrual females was ~300%, thus when the data is combined the amount of CGRP release is ~230%, as shown in Figure 2. When the data from normally cycling females (No OBC) is stratified by days since last menses, dental pulp from women in the week prior to menses evoked the highest amount of 5HT-enhanced CGRP release. These data are interesting as both estrogen and particularly progesterone significantly increase during the luteal phase (Days 16–28) of the menstrual cycle during the week prior to menses [44].
Importantly, there was no significant effect of day since last menses on CGRP release evoked by capsaicin alone, providing further support for a hypothesis that sex steroids specifically affect 5HT modulation of TRPV1 nociceptors. There was an effect of age on 5HT-enhanced CGRP release in dental pulp from females, while there was no effect of age on capsaicin-evoked CGRP release or CGRP release in male dental pulp, which could also indicate an effect of hormonal status in 5HT-enhancement of CGRP release. Our previous studies examining the enhancing effect of 5HT on TRPV1-evoked CGRP release and thermal hyperalgesia were limited to male rats, so whether this effect is observed in female rats is unknown. Preclinical studies examining the effects of 5HT and steroid hormones in female rats across the estrous cycle are warranted to address this possibility.
We also found that 4 different 5HT receptor subtypes known to be involved in 5HT-evoked pain processing [25; 27], 5HT1B, 5HT1D, 5HT2A and 5HT3A receptors, were expressed in male and female human dental pulp. These data provide possible pharmacological targets by which 5HT’s enhancing effects on TRPV1-evoked CGRP release may be controlled. This is important as 5HT receptor expression in the trigeminal system represents a critical target for reducing CGRP release [3; 27], which is correlated with headache and migraine in humans. When quantified, the protein expression of these receptors was comparable between male and female dental pulp. Given our observed alterations of 5HT-enhanced CGRP release over the menstrual cycle, further studies are required to determine if 5HT receptor expression is also altered across the menstrual cycle in human dental pulp. This possibility may be unlikely given that 5HT1 receptor mRNA levels in the mouse trigeminal ganglia do not fluctuate over the estrous cycle [5], however, this may represent an effect that occurs in human but not rodent tissues and should be considered or may not reflect changes in translational control.
Clinical evidence suggests that at least one form of trigeminal pain, headaches and migraine, fluctuates with menstrual cycle status. Headache and migraine typically occur in women around menses and some women only experience migraine associated with menses [33].Considering our data in this population, 5HT may be enhancing CGRP release from the TRPV1 population of trigeminal nociceptors at the onset of menses. Future studies examining whether this effect occurs via TRPV1 and/or via specific 5HT receptors would offer therapeutic insight and are warranted. Importantly, these data illustrate the necessity of examining both male and female subjects in studies of trigeminal pain [18]. Overall, these results indicate that 5HT enhances TRPV1-evoked CGRP release from female human dental pulp and provide evidence of a sexually dimorphic peripheral mechanism modulating trigeminal pain processing. Future studies examining serotonergics that block or attenuate 5HT enhancement of TRPV1-evoked CGRP release may prove therapeutic for elusive trigeminal pain disorders in women.
Synopsis.
5HT enhances TRPV1-evoked CGRP release from female, but not male, human dental pulp. This enhancement occurs greatest during the luteal phase of the menstrual cycle.
Acknowledgments
The authors would like to acknowledge the helpful comments of Michael A. Henry, D.D.S, Ph.D. and the technical assistance of Paul Chen. This work was supported by NIH grants NCATS UL1TR000149 and R01 NS58655 (KMH), T32 DE14318, F32 DE021309 (DRL).
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali Z, Prabhakar H, Rath GP, Dash HH. Enoxaparin induced intracerebral haemorrhage after deep brain stimulation surgery. Eur J Anaesthesiol. 2009;26(7):617–618. doi: 10.1097/eja.0b013e328320a68b. [DOI] [PubMed] [Google Scholar]
- 3.Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain. 2012;153(4):830–838. doi: 10.1016/j.pain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Babenko V, Svensson P, Graven-Nielsen T, Drewes AM, Jensen TS, Arendt-Nielsen L. Duration and distribution of experimental muscle hyperalgesia in humans following combined infusions of serotonin and bradykinin. Brain Res. 2000;853(2):275–281. doi: 10.1016/s0006-8993(99)02270-2. [DOI] [PubMed] [Google Scholar]
- 5.Berman NE, Puri V, Chandrala S, Puri S, Macgregor R, Liverman CS, Klein RM. Serotonin in trigeminal ganglia of female rodents: relevance to menstrual migraine. Headache. 2006;46(8):1230–1245. doi: 10.1111/j.1526-4610.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 6.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci U S A. 1999;96(14):7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LC, Ashcroft DM. Meta-analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache. 2008;48(2):236–247. doi: 10.1111/j.1526-4610.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen SC, Chang TJ, Wu FS. Competitive inhibition of the capsaicin receptor-mediated current by dehydroepiandrosterone in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311(2):529–536. doi: 10.1124/jpet.104.069096. [DOI] [PubMed] [Google Scholar]
- 12.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 13.Ernberg M, Hedenberg-Magnusson B, Kurita H, Kopp S. Effects of local serotonin administration on pain and microcirculation in the human masseter muscle. J Orofac Pain. 2006;20(3):241–248. [PubMed] [Google Scholar]
- 14.Ernberg M, Lundeberg T, Kopp S. Pain and allodynia/hyperalgesia induced by intramuscular injection of serotonin in patients with fibromyalgia and healthy individuals. Pain. 2000;85(1–2):31–39. doi: 10.1016/s0304-3959(99)00233-x. [DOI] [PubMed] [Google Scholar]
- 15.Fehrenbacher JC, Sun XX, Locke EE, Henry MA, Hargreaves KM. Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain. 2009;144(3):253–261. doi: 10.1016/j.pain.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 17.Gassmann M, Grenacher B, Rohde B, Vogel J. Quantifying Western blots: pitfalls of densitometry. Electrophoresis. 2009;30(11):1845–1855. doi: 10.1002/elps.200800720. [DOI] [PubMed] [Google Scholar]
- 18.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargreaves KM. Orofacial pain. Pain. 2011;152(3 Suppl):S25–32. doi: 10.1016/j.pain.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26 (Suppl 3):S12–19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 21.Henry MA, Hargreaves KM. Peripheral mechanisms of odontogenic pain. Dent Clin North Am. 2007;51(1):19–44. v. doi: 10.1016/j.cden.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Herbert MK, Schmidt RF. Activation of normal and inflamed fine articular afferent units by serotonin. Pain. 1992;50(1):79–88. doi: 10.1016/0304-3959(92)90115-R. [DOI] [PubMed] [Google Scholar]
- 23.Herken H, Erdal E, Mutlu N, Barlas O, Cataloluk O, Oz F, Guray E. Possible association of temporomandibular joint pain and dysfunction with a polymorphism in the serotonin transporter gene. Am J Orthod Dentofacial Orthop. 2001;120(3):308–313. doi: 10.1067/mod.2001.115307. [DOI] [PubMed] [Google Scholar]
- 24.Hisaoka T, Morikawa Y, Kitamura T, Senba E. Expression of a member of tumor necrosis factor receptor superfamily, TROY, in the developing olfactory system. Glia. 2004;45(4):313–324. doi: 10.1002/glia.10323. [DOI] [PubMed] [Google Scholar]
- 25.Loyd DR, Chen PB, Hargreaves KM. Antihyperalgesic Effects of Anti-Serotonergic Compounds on Serotonin- and Capsaicin-evoked Thermal Hyperalgesia in the Rat. 2011 doi: 10.1016/j.neuroscience.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loyd DR, Chen PB, Hargreaves KM. Anti-hyperalgesic effects of anti-serotonergic compounds on serotonin- and capsaicin-evoked thermal hyperalgesia in the rat. Neuroscience. 2012;203:207–215. doi: 10.1016/j.neuroscience.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loyd DR, Weiss G, Henry MA, Hargreaves KM. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain. 2011;152(10):2267–2276. doi: 10.1016/j.pain.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu YC, Chen CW, Wang SY, Wu FS. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Ther. 2009;331(3):1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- 29.Luukkainen T, Lahteenmaki P, Toivonen J. Levonorgestrel-releasing intrauterine device. Ann Med. 1990;22(2):85–90. doi: 10.3109/07853899009147248. [DOI] [PubMed] [Google Scholar]
- 30.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Oro-facial pain in the community: prevalence and associated impact. Community Dent Oral Epidemiol. 2002;30(1):52–60. doi: 10.1034/j.1600-0528.2002.300108.x. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Predictors of outcome for orofacial pain in the general population: a four-year follow-up study. J Dent Res. 2004;83(9):712–717. doi: 10.1177/154405910408300911. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ. Orofacial pain: just another chronic pain? Results from a population-based survey. Pain. 2002;99(3):453–458. doi: 10.1016/S0304-3959(02)00181-1. [DOI] [PubMed] [Google Scholar]
- 33.Marcus DA. Interrelationships of neurochemicals, estrogen, and recurring headache. Pain. 1995;62(2):129–139. doi: 10.1016/0304-3959(95)00052-T. [DOI] [PubMed] [Google Scholar]
- 34.Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol. 2006;576(Pt 3):809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106(44):18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;(179):155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 38.Ruparel S, Henry MH, Akopian A, Patil M, Zeldin D, Roman L, Hargreaves KM. Plasticity of cytochrome P450 isozyme expression in rat trigeminal neurons during inflammation. Pain. 2012 doi: 10.1016/j.pain.2012.04.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schabitz WR, Schade H, Heiland S, Kollmar R, Bardutzky J, Henninger N, Muller H, Carl U, Toyokuni S, Sommer C, Schwab S. Neuroprotection by hyperbaric oxygenation after experimental focal cerebral ischemia monitored by MRI. Stroke. 2004;35(5):1175–1179. doi: 10.1161/01.STR.0000125868.86298.8e. [DOI] [PubMed] [Google Scholar]
- 40.Simonetti M, Fabbro A, D’Arco M, Zweyer M, Nistri A, Giniatullin R, Fabbretti E. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain. 2006;2:11. doi: 10.1186/1744-8069-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64–69. [PubMed] [Google Scholar]
- 42.Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517–1523. doi: 10.1212/wnl.55.10.1517. [DOI] [PubMed] [Google Scholar]
- 43.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology. 1994;44(6 Suppl 4):S17–23. [PubMed] [Google Scholar]
- 44.Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 45.Sung D, Dong X, Ernberg M, Kumar U, Cairns BE. Serotonin (5-HT) excites rat masticatory muscle afferent fibers through activation of peripheral 5-HT3 receptors. Pain. 2008;134(1–2):41–50. doi: 10.1016/j.pain.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 46.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430(7001):748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 47.Welch KM. Migraine and ovarian steroid hormones. Cephalalgia. 1997;17 (Suppl 20):12–16. doi: 10.1177/0333102497017S2005. [DOI] [PubMed] [Google Scholar]