Abstract
2-C-methyl-D-erythritol-4-phosphate (MEP) is a key chemical intermediate of the non-mevalonate pathway for isoprenoid biosynthesis employed by many pathogenic microbes. MEP is also the precursor for the synthesis of 4-diphosphocytidyl-2-C-methyl D-erythritol (CDP-ME), another key intermediate of the non-mevalonate pathway. As this pathway is non-existent in higher animals, including humans, it represents great opportunities for novel antimicrobial development. To facilitate the in-depth studies of this pathway, we reported here a formal synthesis of CDP-ME through a new synthesis of 2-C-Methyl-D-erythritol-4-phosphoric acid from D-(+)-arabitol.
Keywords: MEP, CDP-ME, selective phosphorylation, dioxanone, monophosphate
INTRODUCTION
Isoprenoids represent one of the most diverse classes of naturally occurring molecules. To date, there are over 30,000 different compounds identified with representatives involved in cellular processes such as bacterial cell wall biosynthesis, photosynthesis, cell signaling, oxidative phosphorylation and protein biosynthesis.1 All isoprenoids are synthesized from the five-carbon precursors - isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are produced by one of the two biochemical pathways: (a) Mevalonate (MVA) Pathway or (b) Methylerythritol Phosphate (MEP) Pathway. The MVA pathway for isoprenoid biosynthesis is seemingly unrelated to the MEP pathway and is utilized by Archaea, Eukaryea, most Gram-positive bacteria such as Staphylococcus aureus.2–6 In contrast, the MEP pathway is utilized primarily by Gram-negative bacteria (e.g., Pseudomonas aeruginosa, Yersinia pestis), a few Gram-positive bacteria (e.g., Mycobacterium tuberculosis), Chlamydia, Protozoa and some plants.7–18 Genetic disruptions of either biosynthetic pathway are lethal to microbes.19–20 Since the MEP pathway is absent in humans, the enzymes involved in this pathway are ideal targets for drug discovery of novel antimicrobials agents. One of the enzymes that has recently become the subject of intense investigations is 4-diphosphocytidyl-2-C-methyl D-erythritol (CDP-ME) kinase.21–38 This enzyme catalyzes the conversion of CDP-ME to diphosphocytidyl-2-C-methyl D-erythritol-2-phosphate (CDP-MEP).38 Because of this kinase activity, inhibitors of CDP-ME kinase can be explored as novel antimicrobial agents. In order to demonstrate this proof of principle, we have conducted high throughput screening (HTS) for small molecule inhibitors of various microbial CDP-ME kinases.39 In these HTS, large milligram quantity of the substrate, CDP-ME, is required. Therefore, we sought for an efficient and stereoselective synthesis of 2-C-Methyl-D-erythritol-4-phosphoric acid (1), a convenient precursor for the synthesis of CDP-ME.
RESULTS AND DISCUSSION
Recently, other research groups have focused on the synthesis of 2-C-Methyl-D-erythritol-4-phosphoric acid (9% overall yield after 14 steps) 42 or its salts 43,46, 49 because the availability of MEP pathway intermediates and their analogs would substantially assist the ongoing biochemical investigations and the development of novel antibiotics, therapeutic agents and herbicides.40–49 We preferred to synthesize 2-C-Methyl-D-erythritol-4-phosphoric acid (1) for CDP-ME synthesis instead of the its salts because as a free diacid, it could be used directly for CDP-ME synthesis50 without the need for the extra steps for its salt preparation (sodium and trialkylammonium) and of the concerns for the limited solubility of the salts, particularly the sodium salt.51 In our efforts to further simplify the synthesis of 2-C-Methyl-D-erythritol-4-phosphoric acid (1), we chose to explore its synthesis in 7 steps from D-(+)-arabitol (2) instead of the previously reported 14 steps.42
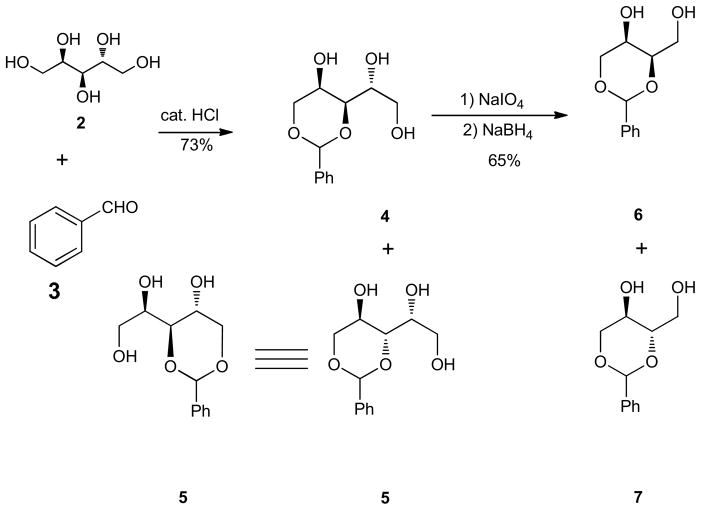
The synthesis of diol 6 (Scheme 1) has been described beginning from the condensation of the benzaldehyde (3) and D-(+)-arabitol (2) in the presence of HCl gas to give a stable benzylidene derivative.46,53 We have modified this procedure by the addition of catalytic HCl (12N) to the mixture of D-(+)-arabitol (2) and benzaldehyde (3). This would allow for the simple laboratory condensation of these reagents and additionally, avoiding the risk of the handling of hazardous HCl gas. Initially, the addition of excess concentrated HCl or liquid H2SO4 caused the formation of undesired side reactions. We soon realized that catalytic amount of HCl (10 mol %), when added to benzaldehyde before the addition of D-(+)-arabitol, afforded a benzylidene derivative as a white solid in good yield (Scheme 1). The purification of the resulting solid mixture was achieved by simply quenching the reaction mixture after 3 days under argon atmosphere with dilute KOH solution (10%) followed by filtration and rinsing of the residue with H2O to afford the resulting triols 4 and 5 in 73% total yield (Scheme 1). Slightly lower yield was obtained after a work-up procedure when the reaction mixture was left to stand for less than a day. Triols 4 and 5 were identified by comparison of NMR data determined from the mixture with literature data.46 Compared to the NMR spectra data reported for the pure product obtained by using HCl gas,46 we have observed the triols 4 and 5 obtained as an equal mixture of inseparable diastereomers when concentrated HCl (12 N) was added (Scheme 1). In the 1H NMR of the diastereomeric mixture, the 1H NMR peaks of the benzylidene acetal hydrogens of the two triols were in equal isomeric ratio at 5.94 ppm and 5.60 ppm and the chemical shift of the desired dioxane 1H NMR peak ((CD3)2CO) at 5.60 ppm was identical to the reported spectral data of triol 4.46 The diastereomeric mixture upon treatment with NaIO4 afforded intermediate aldehyde products which were immediately reduced with NaBH4 to afford an inseparable diastereomeric mixture of diols 6 and 7 in equal isomeric ratio, in a total of 65% yield (Scheme 1). After the NaBH4 reduction, the chemical shift of the dioxane 1H NMR peak at 5.60 ppm of the benzylidene acetal hydrogen for diol 6 did not change from the chemical shift value observed for triol 4 which was in total agreement with the literature data.46
Scheme 1.
Benzylidene formation by catalytic HCl.
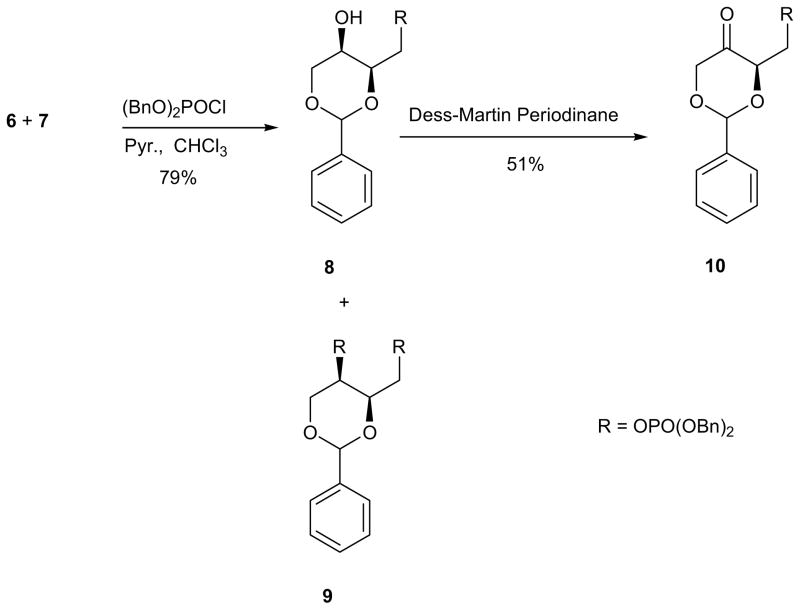
Because diol 6 has both primary and secondary hydroxyl functional groups, we anticipated selective phosphorylation43 of its primary alcohol functionality as the primary hydroxyl group of diol 6 may have greater access to the phosphorylating reagent than its secondary hydroxyl group. In our experiments, the treatment of the equal mixture of diol 6 and 7 with dibenzylphosphorochloridate in the presence of excess pyridine with CH2Cl2 or CHCl3 as a co-solvent, and stirring overnight, caused the significant formation of double phosphorylation product 9 derived from the reaction of diol 6 with dibenzylphosphorochloridate (Scheme 2). However, the primary hydroxyl group in diol 7 was unreactive under these conditions and we were able to isolate the new phosphorylated products from the previous inseparable diol mixture by flash chromatography on silica gel column due to relative polarity differences in the unreactive polar diol 7 and the less polar phosphorylated products 8 and 9 (Scheme 2). The 1H NMR analysis of the crude mixture obtained after the phosphorylation reactions revealed that the acetal 1H NMR peak of diol 6 at 5.60 ppm was transformed to a 1H NMR peak at 5.52 ppm and 5.86 ppm in differential proportions based on the reaction conditions, evident of the formation of two new dioxanes. After purification and further spectral analysis, the acetal 1H NMR peak at 5.52 ppm was assigned to monophosphate 8 and the acetal 1H NMR peak at 5.86 ppm was assigned to bisphosphate 9. Contrary to our expectations, the selectivity of the primary hydroxyl group over the secondary hydroxyl group did not improve even at shorter reaction times (1.5 hrs) in excess pyridine with either co-solvent. However, when the co-solvent was changed to toluene, the monophosphorylated product 8 was formed in 53% yield. Because of the moderate yield and the longer reaction time needed for this reaction, we explored new reaction conditions with CHCl3 since the reaction was usually completed much faster in this solvent.
Scheme 2.
Preparation of dioxanone through a selective monophosphorylation and Dess-Martin periodinane oxidation.
We varied the concentration of pyridine in the reaction mixture to 3, 1 and 0.8 equivalents in CHCl3 and kept the concentration of dibenzylphosphorochloridate to 1 equivalent. While it remained difficult to control the monophosphorylation with 3 equivalents of pyridine, greater selectivity for monophosphate 8 over the undesired bisphosphate 9 was achieved with 1 and 0.8 equivalents of pyridine respectively. The yield of the desired monophosphate 8 improved to 79% with 1 equivalent of pyridine in 2.5 hrs but TLC analysis showed a slight formation of bisphosphate 9. Lower yield of 59% of the mono phosphorylated product was obtained with 0.8 equivalent of pyridine.
Through the continued variation of experimental conditions with regards to the choice of solvents, temperature and time, we successfully achieved a selective phosphorylation of diol 6. Although longer reaction times increased the formation of the double phosphorylated product 9, a major difficulty still lay in the controlling of the amounts of the by-product 9 with bisphosphate moiety even at shorter reaction times. However, the modification of the reaction conditions using chloroform as solvent at 0 °C for 2.5 hrs, allowed the selective formation of the monophosphorylated product 8 in 79% yield as a pure isomer based on unrecovered diol 7. The application of Dess-Martin Periodinane (DMP)52,54 reagent afforded dioxanone 10 in moderate yield of 51% (Scheme 3).
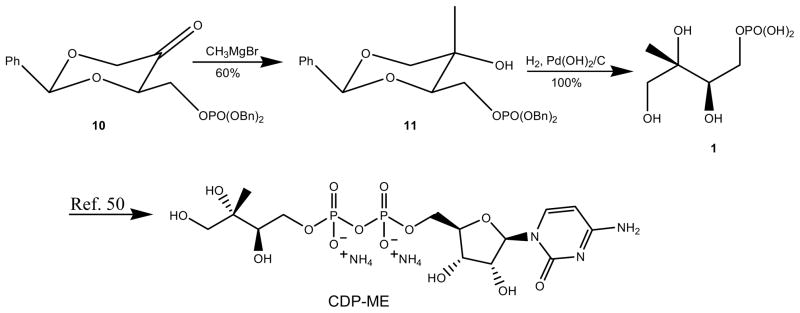
Scheme 3.
Selective alkylation and global deprotection.a
a 1H and 31P NMR spectra of CDP-ME are given in the Supplementary Data.
We envisaged that since our dioxanone monophosphate is a 1,3-dioxan-5-one derivative, its direct methylation would provide access to the tertiary diol 11 as one isomer. When the solution of dioxanone 10 was treated with Grignard reagent at −78 ° C, only one desired isomer was predominantly isolated which was similar to the results obtained from the works of others (Scheme 3).46,49, 52 In the methylation step, the addition of CH3MgBr to dioxanone 10 afforded diol 11 stereoselectively. When the reaction mixture was set at 0.1 M, the occurrence of side reactions caused significant reduction in yields. These side reactions were significantly avoided when the reaction mixture was further diluted to 0.05M. The 1H and 13C NMR spectral data are identical to those previously reported through different routes.46 Although dioxanones bearing bulky α-CH2OR side chains where R is trityl, TBDPS (tert-butyldiphenylsilyl)52 or TBDMS (tert-butyldimethylsilyl)46,49 groups have been shown to have bias for axial nucleophilic attacks, the results of the stereoselectivity on the methylation reactions with phosphate group in the α-CH2OR side chaincorroborates the observation that all R groups in the α-CH2OR side chain in 1,3-diaxan-5-ones have the same preference for axial nucleophilic attacks.52
The hydrogenolysis of compound 11 was conducted in MeOH under hydrogen atmosphere (balloon) with vigorous stirring for 9 hours. After the completion of the hydrogenolysis, phosphoric acid 1 was isolated in quantitative yield. The 1H and 13C spectral data are in agreement with previous reports55 and used for CDP-ME synthesis without further purification. The final CDP-ME synthesis was carried out by the experimental conditions developed by Eoh, H. et al. The 1H and 31P NMR spectral data of synthesized CDP-ME were identical to those reported.50–51
CONCLUSION
We have achieved a stereoselective synthesis of MEP in 7 steps from D-(+)-arabitol in 11.5% overall yield. Our initial synthetic strategy embodies the application of catalytic HCl for the preparation of the protected benzylidene derivative needed as the key starting material for the synthesis of phosphoric acid 1. The desired diol in a diastereomeric diol mixture was selectively converted to a monophosphate through controlled reaction conditions. 2-C-Methyl-D-erythritol-4-phosphoric acid (1) was obtained after the subsequent oxidation of the monophosphate with Dess-Martin Periodinane, selective alkylation of the resulting dioxanone with CH3MgBr and hydrogenolysis.
EXPERIMENTAL SECTION
General Experimental Procedures. Anhydrous tetrahydrofuran (THF), toluene and pyridine were obtained from commercial vendors and used without further purification. Dichloromethane was distilled freshly from CaH2. Methanol, hexane and EtOAc were used directly without further purification. All non-aqueous reactions were done under an argon atmosphere, in oven-dried or flame-dried glassware and with magnetic stirring. Flash chromatography was done on silica gel with an average 63–200 μm particle size. The 1H, 13C and 31P NMR spectra were recorded at 400 MHz with CDCl3, DMSO, or D2O as solvents. High-resolution and electrospray (ES) mass spectra were obtained at the University of Utah Mass Spectrometry Facility.
(1R)-1-((4R,5R)-5-hydroxy-2-phenyl-1,3-dioxan-4-yl)ethane-1,2-diol (4) + (1R)-1-((4S,5R)-5-hydroxy-2-phenyl-1,3-dioxan-4-yl)ethane-1,2-diol (5)
The benzylidene protection was modified from literature procedure.46 In a dry round-bottomed flask containing benzaldehyde (8.5 mL, 84.45 mmol), HCl (0.59 mL (12N), 7.1 mmol) was added and stirred. This was followed by the immediate addition of D-(+)-arabitol (10.71g, 70.38 mmol) in spatula batches. The mixture was stirred briefly and left to stand for 3 days under argon. The resulting white solid was scraped out of the flask and quenched with dilute KOH (10%, 20mL). The remaining solid particles were further crushed in the KOH mixture, filtered and washed with distilled H2O. After drying, the mixture was obtained as a white solid (12.38g, 73%).
(4R,5R)-4-(hydroxymethyl)-2-phenyl-1,3-dioxan-5-ol (6) + (4S,5R)-4-(hydroxymethyl)-2-phenyl-1,3-dioxan-5-ol (7)
The solid mixture of 4 + 5 (12.38g, 51.5 mmole, 1: 1 isomer ratio) was added to a flask containing CH2Cl2 (150 mL) and satd. NaHCO3 (50 mL) followed by the addition of NaIO4 (22g, 103 mmol). The mixture was stirred for 1.5 hrs and filtered. After thorough rinsing of the residue with EtOAc, the filtrate was separated and the aqueous layer was extracted with EtOAc. The combined organic layer was washed with brine, dried (MgSO4) and concentrated in vacuo. The crude oil was dissolved in MeOH (200 mL) and brought to 0 °C. NaBH4 (2.34g, 61.80 mmol) was added in small portions slowly to the cold solution of the crude compound in MeOH. The mixture was then allowed to stir for 2 hrs and quenched with satd. NH4Cl. The mixture was evaporated overnight. The resulting white crude mixture was dissolved in distilled H2O and extracted with EtOAc. The combined organic layer was washed with brine, dried (MgSO4) and concentrated in vacuo and purified by flash Chromatography (1:1 hexane: EtoAc, 3:7 acetone:CHCl3) to afford diastereomeric mixture of alcohol 6 and 7 as a white solid (7.0 g, 65%).
Dibenzyl (((4R,5R)-5-hydroxy-2-phenyl-1,3-dioxan-4-yl)methyl) phosphate (8)
Liquid (BnO)2POH (6.12 mL, 27.08 mmol) was dissolved in dry toluene (70 mL) followed by the addition of N-chlorosuccinimide (4.02 g, 30.08 mmol).56 The mixture was stirred for 2 hrs and filtered. The residue was washed with toluene followed by the concentration of the filtrate. The resulting concentrated oil was then added to the solution of alcohol 6 and 7 (5.27g, 25.07 mmol, isomer ratio 1:1) in CHCl3 (50 mL) and brought to 0 °C. Pyridine (2.03 mL, 25.07 mmol) was added dropwise and slowly. The mixture was allowed to stir for 2.5 hrs and quenched with satd. NH4Cl. After separation, aqueous layer was extracted with EtOAc. The combined organic layer was washed with brine, dried and concentrated in vacuo. The crude oil was purified by flash chromatography (2:1, 3:2 hexane:EtOAc) to afford monophosphate 8 as a white solid (4.7g, 79%) based on 50% of unrecovered starting material).1H NMR (400 MHz, CDCl3): δ = 7.47–7.25 (m, 15H), 5.52 (s, 1H), 5.10 (m, 4H), 4.22–3.99 (m, 6H). (Presence of impurity peaks in the 1H NMR spectrum at δ = 0.8. 1.2, 2.1and 3.5 ppm),; 13C NMR (100 MHz, CDCl3): δ = 137.3, 135.7, 135.6, 129.2 – 128.0 (aromatic C), 125.9, 101.3, 77.9 (d, JCP = 6.9 Hz), 77.2, 72.3, 69.5 (d, JCP = 5.4 Hz), 66.2 (d, JCP = 5.4 Hz), 63.1; 31P NMR (D2O): δ = 0.43 (s); HRMS (ESI/FTMS) calcd for M+H of C25H27O7P, 471.15; found, 471.16.
Bisphosphonate 9
Liquid (BnO)2POH (7.75 mL, 35.07 mmol) was dissolved in dry toluene (150 mL) followed by the addition of N-chlorosuccinimide (5.15 g, 38.57 mmol).56 The mixture was stirred for 2 hrs and filtered. The residue was washed with toluene (20 mL) followed by the concentration of the filtrate. The resulting concentrated oil was then added to the solution of alcohol 6 and 7 (2.95 g, 14.03 mmol, isomer ratio 1:1) in CHCl3 (25 mL) and brought to 0 °C. Pyridine (3.40 mL, 42.08 mmol) was added dropwise. The mixture was allowed to stir 24 hrs and quenched with satd. NH4Cl. After separation, aqueous layer was extracted with EtOAc. The combined organic layer was washed with brine, dried and concentrated in vacuo. The crude oil was purified by flash chromatography (1:1 hexane:EtOAc; 2:2:1 hexane:EtOAc:MeOH) to afford diphosphosphate 9 as a clear oil (6.73g, 66%). 1H NMR (400 MHz, CDCl3): δ = 7.47–7.29 (m, 25H), 5.86 (s, 1H), 5.10–4.95 (m, 8H), 4.12–4.02 (m, 6H); 13C NMR (100 MHz, CDCl3): δ = 136.5, 135.5 (2C), 129.4, 128.5–127.8 (aromatic C), 126.5, 104.2, 76.2–66.2 (8C) (76.1, dd, JCP = 20.3Hz, 8.4 Hz), (69.4, t, JCP = 5.4 Hz), (66.3, dd, JCP = 12.3 Hz, 5.4 Hz); 31P NMR (D2O): δ = 0.25 (s), 0.04 (s); HRMS (ESI/FTMS) calcd for M+H of C39H40O10P2, 731.22; found, 731.22.
Dibenzyl (((4R)-5-oxo-2-phenyl-1,3-dioxan-4-yl)methyl) phosphate (10)
Dess-Martin Periodinane reagent (2.36g, 5.56 mmol) was added to the solution of alcohol 8 (2.18g, 4.64 mmol) in CH2Cl2 (50 mL) and stirred for 2 hrs. The mixture was diluted with ether (100 mL) quenched with a mixture of satd. NaHCO3 (30 mL) and Na2S2O3 (ca. 5g) and stirred for about 5 mins until the white mixture was clear. After separation, aqueous layer was extracted with ether and the combined organic layer was washed with brine, dried (MgSO4) and concentrated in vacuo. The resulting crude mixture was purified by flash chromatography (2:1, 1:1 hexane:EtoAc) to afford dioxalone 10 as a clear oil (1.11g, 51%).
1H NMR (400 MHz, CDCl3): δ = 7.53–7.25 (m, 15H), 5.94 (s, 1H), 5.04 (d, J = 9.6 Hz, 2H), 5.00 (d, J = 7.2 Hz, 2H), 4.63 (m, 1H), 4.50–4.38 (m, 4H),; 13C NMR (100 MHz, CDCl3): δ = 202.4, 136.4, 135.6, 135.4, 129.3, 128.5–127.8 (aromatic C), 126.1, 99.2, 81.1 (d, JCP = 6.8 Hz), 72.2, 69.3 (d, JCP = 6.1 Hz), 65.5 (d, JCP = 5.4 Hz).; 31P NMR (D2O): δ = −0.19; HRMS (ESI/FTMS) calcd for M+H of C25H25O7P, 469.14; found 469.14.
Dibenzyl (((4R,5S)-5-hydroxy-5-methyl-2-phenyl-1,3-dioxan-4-yl)methyl) phosphate (11)
The solution of dioxalone 10 (1.86 g, 3,97 mmol) in THF (40 mL) was brought to − 78 °C, CH3MgBr (3.0 mL, 11.91 mmol) was added dropwise. The mixture was stirred for 1 hr and quenched slowly with satd. NH4Cl. After separation, the aqueous layer was extracted with EtOAc and washed with brine, dried (MgSO4) and concentrated in vacuo. The resulting crude mixture was purified by flash chromatography (3:2, 1:1 hexane:EtOAc) to afford alcohol 11 (1.12g, 60%). 1H and 13C NMR spectral data are the same as those published.46
2-C-methylerythritol 4-phosphate (1)
Solid Pd(OH)2/C (180 mg) was added to the solution of alcohol 11 (455 mg, 0.94 mmol) in MeOH (30 mL) under H2 gas (balloon) and stirred vigorously for 9 hrs. The mixture was filtered followed by the rinsing of the filter cake with MeOH (50 mL). The filtrate was concentrated and dried in vacuo to afford compound 1 (203 mg, 100%). 1H and 31P NMR spectral data are the same as published.55
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (5R01 HD054744-04 and 3R01 HD054744-04S1) (to KL).
Footnotes
1H, 13C NMR of compound 8, 9, 10 and 1H, 31P NMR of CDP-ME are available in the online version
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bochar, et al. D. A. Biosynthesis of mevalonic acid from acetyl-CoA. In: Cane D, editor. Comprehensive Natural Products Chemistry. Pergamon Press; Oxford, Britain: 1999. pp. 14–44. [Google Scholar]
- 2.Balibar CJ, Shen X, Tao J. J Bacteriol. 2009;191:851–61. doi: 10.1128/JB.01357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elson CE. J Nutr. 1995;125(6 Suppl):1666S–1672S. doi: 10.1093/jn/125.suppl_6.1666S. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, Brown MS. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 5.Smit A, Mushegian A. Genome Res. 2000;10:1468–84. doi: 10.1101/gr.145600. [DOI] [PubMed] [Google Scholar]
- 6.Swanson KM, Hohl RJ. Curr Cancer Drug Targets. 2006;6:15–37. doi: 10.2174/156800906775471743. [DOI] [PubMed] [Google Scholar]
- 7.Dubey VS, Bhalla R, Luthra R. J Biosci. 2003;28:637–46. doi: 10.1007/BF02703339. [DOI] [PubMed] [Google Scholar]
- 8.Eisenreich W, et al. Cell Mol Life Sci. 2004;61:1401–26. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirai N, et al. Biosci Biotechnol Biochem. 2000;64:1448–58. doi: 10.1271/bbb.64.1448. [DOI] [PubMed] [Google Scholar]
- 10.Hunter WN. J Biol Chem. 2007;282:21573–7. doi: 10.1074/jbc.R700005200. [DOI] [PubMed] [Google Scholar]
- 11.Hunter WN, et al. Biochem Soc Trans. 2003;31:537–42. doi: 10.1042/bst0310537. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenthaler HK, et al. Z Naturforsch C. 2000;55:305–13. doi: 10.1515/znc-2000-5-601. [DOI] [PubMed] [Google Scholar]
- 13.Mueller C, et al. Biochem Soc Trans. 2000;28:792–3. [PubMed] [Google Scholar]
- 14.Nagata N, et al. Planta. 2002;216:345–50. doi: 10.1007/s00425-002-0871-9. [DOI] [PubMed] [Google Scholar]
- 15.Paseshnichenko VA. Biochemistry (Mosc) 1998;63:139–48. [PubMed] [Google Scholar]
- 16.Rohdich F, et al. Curr Opin Chem Biol. 2001;5:535–40. doi: 10.1016/s1367-5931(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 17.Seto H. Tanpakushitsu Kakusan Koso. 1997;42:2590–600. [PubMed] [Google Scholar]
- 18.Zeidler J, et al. Biochem Soc Trans. 2000;28:796–8. [PubMed] [Google Scholar]
- 19.Testa CA, Cornish RM, Poulter CD. J Bacteriol. 2004;186:473–80. doi: 10.1128/JB.186.2.473-480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilding EI, et al. J Bacteriol. 2000;182:4319–27. doi: 10.1128/jb.182.15.4319-4327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanasamy P, Eoh H, Brennan PJ, Crick DC. Chem Biol. 2010;17:117–122. doi: 10.1016/j.chembiol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eoh H, Narayanasamy P, Brown AC, Parish T, Brennan PJ, Crick DC. Chem Biol. 2009;16:1230–1239. doi: 10.1016/j.chembiol.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanasamy P, Eoh H, Crick DC. Tetrahedron Lett. 2008;49:4461–4463. doi: 10.1016/j.tetlet.2008.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sgraja T, Alphey MS, Ghilagaber S, Marquez R, Robertson MN, Hemmings JL, Lauw S, Rohdich F, Bacher A, Eisenreich W, Illarionova V, Hunter WN. FEBS J. 2008;275:2779–2794. doi: 10.1111/j.1742-4658.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn CS, Pai H. Plant Mol Biol. 2008;66:503–517. doi: 10.1007/s11103-007-9286-0. [DOI] [PubMed] [Google Scholar]
- 26.Eoh H, Brown AC, Buetow L, Hunter WN, Parish T, Kaur D, Brennan PJ, Crick DC. J Bacteriol. 2007;189:8922–8927. doi: 10.1128/JB.00925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi W, Feng J, Zhang M, Lai X, Xu S, Zhang X, Wang H. J Biochem Mol Biol. 2007;40:911–920. doi: 10.5483/bmbrep.2007.40.6.911. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh M, Goodman HM. Planta. 2006;223:779–784. doi: 10.1007/s00425-005-0140-9. [DOI] [PubMed] [Google Scholar]
- 29.Lherbet C, Pojer F, Richard SB, Noel JP, Poulter CD. Biochemistry. 2006;45:3548–3553. doi: 10.1021/bi0520075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Testa CA, Lherbet C, Pojer F, Noel JP, Poulter CD. Biochim Biophys Acta, Proteins Proteomics. 2006;1764:85–96. doi: 10.1016/j.bbapap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Bernal C, Mendez E, Terencio J, Boronat A, Imperial S. Anal Biochem. 2005;340:245–251. doi: 10.1016/j.ab.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Bernal C, Palacin C, Boronat A, Imperial S. Anal Biochem. 2005;337:55–61. doi: 10.1016/j.ab.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Richard SB, Lillo Antonietta M, Tetzlaff CN, Bowman ME, Noel JP, Cane DE. Biochemistry. 2004;43:12189–12197. doi: 10.1021/bi0487241. [DOI] [PubMed] [Google Scholar]
- 34.Miallau L, Alphey MS, Kemp LE, Leonard GA, McSweeney SM, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN. Proc Natl Acad Sci U S A. 2003;100:9173–9178. doi: 10.1073/pnas.1533425100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada T, Kuzuyama T, Satoh S, Kuramitsu S, Yokoyama S, Unzai S, Tame JRH, Park SJ. Biol Chem. 2003;278:30022–30027. doi: 10.1074/jbc.M304339200. [DOI] [PubMed] [Google Scholar]
- 36.chuhr CA, Hecht S, Kis Klaus Eisenreich W, Wungsintaweekul J, Bacher A, Rohdich F. Eur J Org Chem. 2001;17:3221–3226. doi: 10.1021/jo0100300. [DOI] [PubMed] [Google Scholar]
- 37.Kemp LE, Bond CS, Hunter WN. Acta Crystallogr, Sect D: Biol Crystallogr. 2001;D57:1189–1191. doi: 10.1107/s0907444901010137. [DOI] [PubMed] [Google Scholar]
- 38.Tang M, Odejinmi SI, Allette YM, Vankayalapati H, Lai K. Bioorg Med Chem. 2011;19:5886–5895. doi: 10.1016/j.bmc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesner J, Hintz M, Altincicek B, Sanderbrand S, Weidemeyer C, Beck E, Jomaa H. Exp Parasitol. 2000;96:182–186. doi: 10.1006/expr.2000.4566. [DOI] [PubMed] [Google Scholar]
- 40.Kuzuyama T, Takahashi S, Watanabe H, Seto H. Tetrahedron Lett. 1998;39:4509–4512. [Google Scholar]
- 41.Koppisch AT, Blagg BSJ, Poulter CD. Org Lett. 2000;2:215–217. doi: 10.1021/ol991299x. [DOI] [PubMed] [Google Scholar]
- 42.Kis K, Wungsintaweekul J, Eisenreich W, Zenk MH, Bacher A. J Org Chem. 2000;65:587–592. doi: 10.1021/jo9905118. [DOI] [PubMed] [Google Scholar]
- 43.Hoeffler JF, Pale-Grosdemange C, Rohmer M. Tetrahedron. 2000;56:1485–1489. [Google Scholar]
- 44.Fontana A. J Org Chem. 2001;66:2506–2508. doi: 10.1021/jo005732o. [DOI] [PubMed] [Google Scholar]
- 45.Koppisch AT, Poulter CD. J Org Chem. 2002;67:5416–5418. doi: 10.1021/jo025736o. [DOI] [PubMed] [Google Scholar]
- 46.Urbansky M, Davis CE, Surjan JD, Coates RM. Org Lett. 2004;6:135–138. doi: 10.1021/ol0362562. [DOI] [PubMed] [Google Scholar]
- 47.Raghavan S, Sreekanth T. Tetrahedron Lett. 2007;48:9090–9092. [Google Scholar]
- 48.Koumbis AE, Kotoulas SS, Gallos JK. Tetrahedron. 2007;63:2235–2243. [Google Scholar]
- 49.Lagisetti C, Urbansky M, Coates RM. J Org Chem. 2007;72:9886–9895. doi: 10.1021/jo0711900. [DOI] [PubMed] [Google Scholar]
- 50.Eoh H, Narayanasamy P, Brown AC, Parish T, Brennan PJ, Crick DC. Chem Biol. 2009;16:1230–1239. doi: 10.1016/j.chembiol.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koppisch AT, Poulter CD. J Org Chem. 2002;67:5416–5418. doi: 10.1021/jo025736o. [DOI] [PubMed] [Google Scholar]
- 52.Carda M, Casabó P, González F, Rodríguez S, Domingo LR, Marco JA. Tetrahedron: Asymmetry. 1997;8:559–577. [Google Scholar]
- 53.Haskins WT, Hann RM, Hudson CS. J Am Chem Soc. 1943;65:1663–1667. [Google Scholar]
- 54.Dess DB, Martin JC. J Org Chem. 1983;48:4155–4156. [Google Scholar]
- 55.Kis K, Wungsintaweekul J, Eisenreich W, Zenk MH, Bacher A. J Org Chem. 2000;65:587–592. doi: 10.1021/jo9905118. [DOI] [PubMed] [Google Scholar]
- 56.Gao F, Yan X, Shakya T, Baettig OM, Brunet SAM, Berghuis AM, Wright GD, Auclair K. J Med Chem. 2006;49:5273–5281. doi: 10.1021/jm060732n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.