Figure 2.
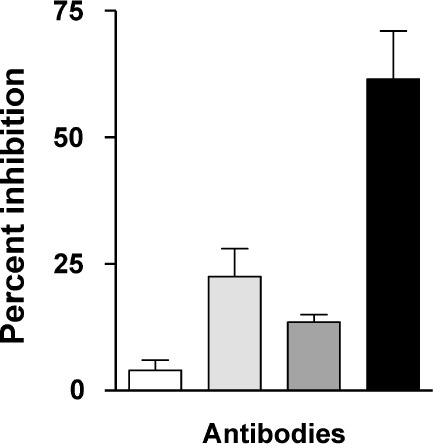
(□) Control. ( ) B24 only. (
) B24 only. ( ) 114-9C10 only. (▪) B24 + 114-9C10. A combination of antibodies mediates synergistic virus neutralization. Mice were vaccinated with a single HIV-1IIIB–derived envelope or with a combination of envelopes including envelope from HIV-1IIIB. Delivery vehicles included recombinant DNA, vaccinia virus, and protein. Splenic cells from vaccinated mice were then harvested for B-cell hybridoma production. Once stable hybridomas (B24 and 114-9C10) were derived and cloned, respective antibodies were harvested from hybridoma culture media and purified by affinity chromatography with protein G sepharose. The neutralization assay was initiated by incubating HIV-1IIIB (approximately 10 TCID-50 per well in a 96-well microtiter plate) with or without antibodies in R10 medium (RPMI 1640 plus 10% heat-treated fetal bovine serum, penicillin, streptomycin and 4mM glutamine). Antibodies were used at concentrations selected to yield fractional virus inhibition, in order to measure additive or synergistic effects (final concentrations of B24 and 114-9C10 were 0.05 μg/mL and 10 μg/mL, respectively). After overnight incubation, the contents of wells were transferred to confluent GHOST-CXCR4 cells in 96-well plates and incubated overnight. Cells were washed with R10 medium and incubated an additional 3 days, after which supernatants were analyzed for virus growth with a Coulter HIV-1 p24 antigen assay (Beckman-Coulter, Miami, FL). Percent inhibition was determined by comparing test wells with wells containing virus and no antibody. Control wells were with virus and HIV-1 negative human serum.
) 114-9C10 only. (▪) B24 + 114-9C10. A combination of antibodies mediates synergistic virus neutralization. Mice were vaccinated with a single HIV-1IIIB–derived envelope or with a combination of envelopes including envelope from HIV-1IIIB. Delivery vehicles included recombinant DNA, vaccinia virus, and protein. Splenic cells from vaccinated mice were then harvested for B-cell hybridoma production. Once stable hybridomas (B24 and 114-9C10) were derived and cloned, respective antibodies were harvested from hybridoma culture media and purified by affinity chromatography with protein G sepharose. The neutralization assay was initiated by incubating HIV-1IIIB (approximately 10 TCID-50 per well in a 96-well microtiter plate) with or without antibodies in R10 medium (RPMI 1640 plus 10% heat-treated fetal bovine serum, penicillin, streptomycin and 4mM glutamine). Antibodies were used at concentrations selected to yield fractional virus inhibition, in order to measure additive or synergistic effects (final concentrations of B24 and 114-9C10 were 0.05 μg/mL and 10 μg/mL, respectively). After overnight incubation, the contents of wells were transferred to confluent GHOST-CXCR4 cells in 96-well plates and incubated overnight. Cells were washed with R10 medium and incubated an additional 3 days, after which supernatants were analyzed for virus growth with a Coulter HIV-1 p24 antigen assay (Beckman-Coulter, Miami, FL). Percent inhibition was determined by comparing test wells with wells containing virus and no antibody. Control wells were with virus and HIV-1 negative human serum.
