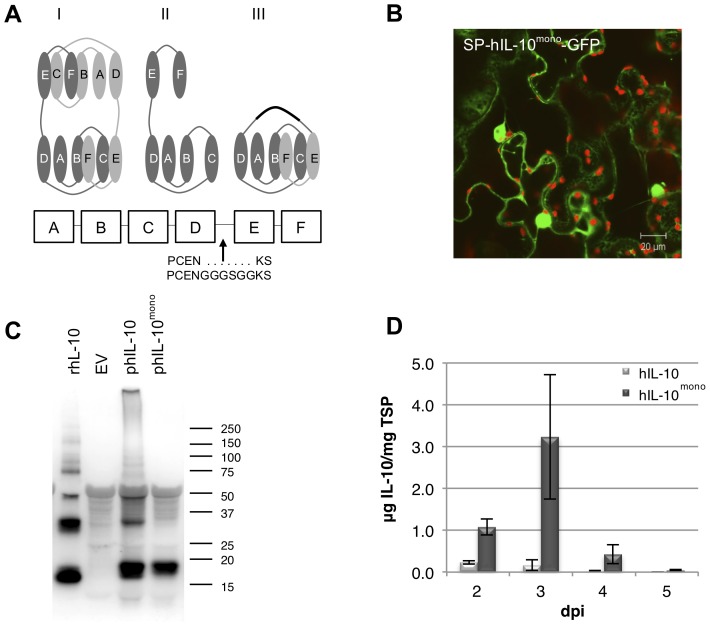
Figure 5. Analysis of expression of a stable monomeric form of human IL-10.
A stable monomeric form of human IL-10 (hIL-10mono) does not granulate and yield increases 30-fold. (A) Three cartoons illustrating the human IL-10 (I) dimer, (II) monomer and (III) stable monomer structure, as well as a schematic representation of the human (h) IL-10 alpha helices A–F. Helices are represented by ovals, whereby a fragment of the amino acid sequence and the location of insertion of the small GS-linker is indicated. (B) Whole mount confocal microscopy output of GFP fused C-terminally to hIL-10mono including native signal peptide (SP). (C) Western blot analysis under non-reducing conditions of plant produced hIL-10 and hIL-10mono. As controls, empty vector (EV) and 50 ng recombinant (r) E. coli produced hL-10 were used. A molecular weight marker is indicated in kDa. (D) Yield of hIL-10 and hIL-10mono in crude extracts 2 to 5 days post infiltration as determined by ELISA (n = 3, error bars indicate standard error). Average yield of hIL-10mono was significantly higher compared to hIL-10.