Fig. 1.
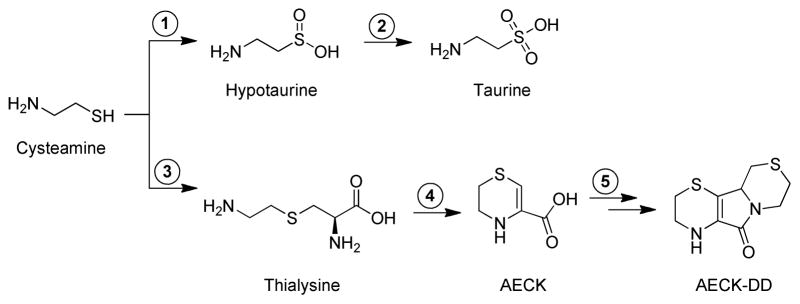
Theoretical pathways for cysteamine metabolism. In the upper pathway, cysteamine is oxidized to hypotaurine by a highly specific cysteamine dioxygenase (step 1) [1,2]. In the second step, hypotaurine is oxidized to taurine. It is not yet clear whether the second step is enzyme catalyzed [1,2]. In the lower pathway, proposed by Cavallini and colleagues [3–17], the first step (step 3) is catalyzed by cystathionine β-synthase. In this reaction, cysteamine is postulated to replace L-homocysteine as a substrate [i.e., cysteamine + L-serine → L-thialysine + H2O]. In agreement with this hypothesis it was later shown by Shen et al. that human cystathionine β-synthase catalyzes an efficient β replacement reaction with L-serine and cysteamine [18]. Thialysine (aminoethyl-L-cysteine) is transaminated to the corresponding α-keto acid, which cyclizes to form a six-membered ring containing an internal ketimine (aminoethyl cysteine ketimine, AECK) (step 4). The ketimine form of AECK is in tautomeric equilibrium with an enamine structure. The latter predominates at neutral pH [6], and is shown in the figure. AECK dimerizes and oxidizes with the loss of one CO2 per dimer to generate aminoethyl cysteine ketimine decarboxylated dimer (AECK-DD) presumably through non-enzymatic reactions (step 5).