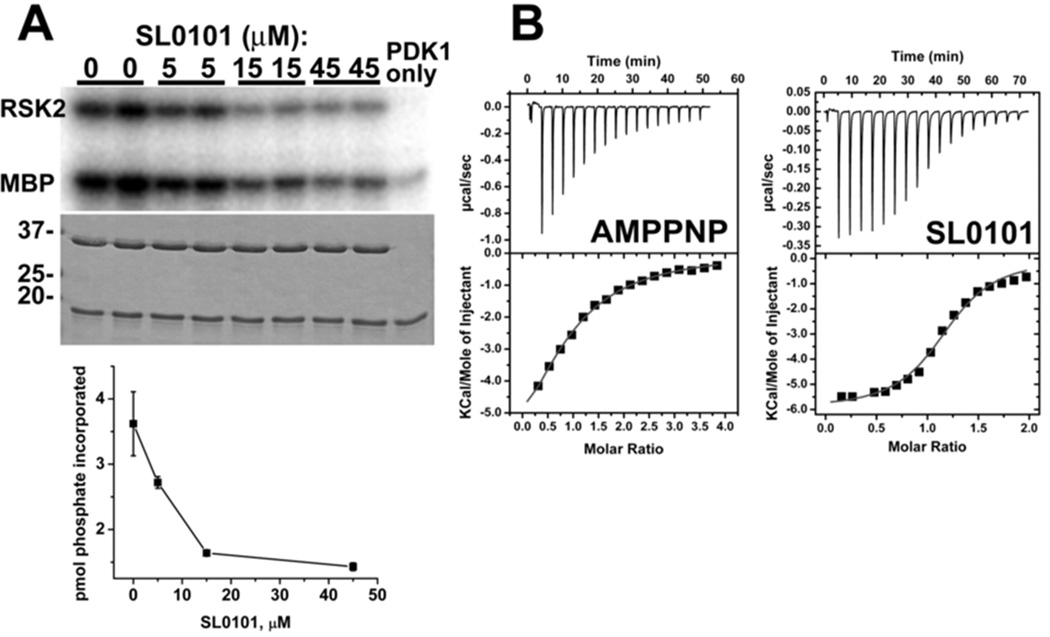
Figure 2.
Functional properties of the recombinant mRSK2NTKD.
(A) Inhibition by SL0101 of the catalytic activity of the phosphorylated protein. The kinase assays were performed in the presence of indicated amounts of SL0101 using myelin basis protein (MBP) as a substrate. Incorporated phosphate was analyzed by autoradiography of gel samples and proteins were visualized by Coomassie Blue staining (shown beneath corresponding autoradiography images). Lower panel: kinase activity data (averaged from duplicate measurements) were expressed as picomoles of incorporated phosphate and plotted against SL0101 concentration. The decreasing amount of phosphorylated mRSK2NTKD following incubation with SL0101 suggests that autophosphorylation of the kinase is also subject to inhibition by SL0101.
(B) ITC profiles of the interaction of AMP-PNP and SL0101 with wild type mRSK2NTKD. The individual dissipated heats were plotted against the molar ratio of interacting partners (squares) to estimate thermodynamic parameters. The data were fitted with a “one set of sites model” shown as a solid red line on lower panels. The best fits resulted in Kd = 50 µM, n = 0.85, ΔH = −9.1 kcal/mol and ΔS = −10.9 cal/mol/deg (TΔS = −3.2 kcal/mol) for AMP-PNP (ΔG = −5.9 kcal/mol) ; and Kd = 2.9 µM, n = 1.16, ΔH = −5.9 kcal/mol and ΔS = 4.9. cal/mol/deg (TΔS = 1.4 kcal/mol) for SL0101(ΔG = − 7.3 kcal/mol). Only representative experiments are shown out of several, which all yielded reproducible results.