Abstract
Clinical and histopathological results of the hyaluronic acid skin substitute treatment of the patients who admitted to Inonu University Medical Faculty Plastic Reconstructive and Aesthetic Surgery clinic between january 2011 and march 2012 were evaluated. The patients were divided into two groups. HA were used for treatment of Hypertrophic scar (HS) or Keloid (K) in 10 patients of the first group. Skin biopsies obtained at peroperative and postoperative 3rd month were subjected to histopathologic examination in this group. In the second group, 10 patients with full thickness soft tissue loss secondary to burns, trauma or excisional reasons were also treated with HA application. Vancouver scar scale were used to determine the scar quality in both groups. Mean age was 25. 2 ± 10.2 and mean follow-up duration was 6.3±3.6 months in group 1. Preoperative and postoperative VSS scores in group 1 were 10.7±1.16 and 6.2±0.91, respectively. This difference was statistically significant (p<0,005). No HS or K development was seen in any patient in group 2 during the following period. Collagenisation scores of preoperative skin biopsies were significantly higher than postoperative scores (p<0,0001).Vascularisation scores of preoperative skin biopsies were significantly lower than postoperative scores (p<0,00001). The use of HA skin substitute in adults for treatment of HS or K provided the desired clinical healing in the 6 months’ follow-up periods. At the same time, HA application as an alternative to other treatment modalities led to a durable skin coverage in full thickness tissue loss in adult patients.
Keywords: Hypertrophic scar, hyalomatrix, wound healing
Introduction
Hypertrophic scarring (HS) usually occurs within 4 to 8 weeks following wound infection, wound closure with excessive tension or other traumatic skin injury. Many factors such as race, age, genetic factors, hormone levels, atopy and immunologic responses of the individual patient appear to play a role. Type of injury, wound size, depth, anatomical region and mechanical tension on wound are important as well. The lack of dermis results in severe contraction and HS especially in regions around joints [1]. Management of HS and keloids (K) has advanced from crude, invasive methods such as gross excision and radiation to intralesional or topical agents that act on a cellular level. There is no universally accepted treatment regimen and no evidence-based literature to guide management. Novel therapies deserve further investigation but remain without proven benefit to date.
Surgical treatment of injuries with skin loss has improved with the advent of regenerative medicine and use of skin subtitutes. Nowadays, dermal substitutes are considered to play a more prominent role in burn surgery and have shown to minimize HS, contractures and increase scar elasticity in acute burn wounds [2]. Several studies have shown good results with the use of dermal substitution in acute and reconstructive wounds [3,4]. The use of dermal substitutes in acute and reconstructive wounds is thought to lead to better scar function and appearance. As a skin substitute Hyalomatrix PA (Fidia Advanced Biopolymers, Abano Terme, Italy) is a synthetic acellular dermal analog and used for full thickness skin defects. It is a bioresorbable dermal substitute made of HYAFF, a long derivative of hyaluronic acid. Hyaluronic acid (HA) has been found to be useful in different medical areas, like wound healing and osteoarthritic joint treatment [5,6]. Although it was firstly regarded as an interfilling material, HA functions are still the subject of ongoing research. The glycosaminogylcan, HA also known as hyaluronan can be found in many tissues and body fluids, with the highest concentration in connective tissue and skin. In the early inflammation, HA accumulates in the wound tissue and interacting with CD44 induces proinflammatory cytokines and enhances cell infiltration. The HA content in fetal wound remaining high for larger periods than in adults and the corresponding hyaluronidase levels being relatively low lead to the suggestion that HA may at least in part, reduce collagen deposition and scarring [7]. In this study, We present clinical and histopathological late results of treatment keloid and HS excision with following HA application in a serie of 10 adult patients. We present also our clinical experience of 10 adult patients with soft tissue defects either posttraumatic or postexcisional.
Materials and methods
Between 2010 and 2012, 10 adult patients with HS and K were enrolled to the study. They were informed about the treatment protocol and their approvals were obtained. Local ethic committee approved the the study (Institutional Ethical approval number 2011/39). Table 1 shows demographic data of the patients. They all seeked for therapy for cosmetic and/ or functional problems. An informed consent which included a punch skin biopsy at the third postoperative month was obatined from each patient of the group 1.
Table 1.
Demographics in group1 patients
| Patient/age/sex | Follow-up | Localisation | Etiology | Scar type |
|---|---|---|---|---|
| HO/18years/male | 1 year | Left ankle | Electrical burn | HS |
| SŞ/23 years/male | 9 months | Right wrist | Scald burn | HS |
| SU/17 years/female | 6 months | Right forearm | Scald burn | K |
| SA/21 years/male | 6 months | Right palm | Scald burn | HS |
| FK/51years/female | 3 months | Left palm | Contact burn | HS |
| MY/31 years/male | 6 months | Right palm | Scald burn | HS |
| AG/28years/male | 3 months | Right shoulder | postvaccineal | K |
| AB/26years/male | 6 months | Left antecubital | posttraumatic | HS |
| EE/18 years/female | 3 months | Left tight | Posttraumatic | HS |
| YR/19 years/male | 3 months | servical | Flame burn | K |
After the surgical therapy, they were followed for at least 6 months (ranging between 6 months and 1 year) and clinical photos for each patient were recorded. At 3rd postoperative month, a skin punch biopsy were taken from the treated areas of the patients for histopathologic comparision with the preoperative skin excisional biopsies.
Treatment protocol
Pathologic scar tissue was marked with skin marker. Then under general anesthesia the scar tissue was removed with lefting minute remnants at the edges of the excison following to skin disinfection with topical povidone iodine. After having full hemostasis, esterified HA (Hyalomatrix PA, HYAFF, Fidia Italy) applied to the defect with stapler or sutures. The patient was regularly controlled at 10 days interval for infection. At the 28th postoperative day, a split-thickness skin grafting was performed. When epithelization completed, the patients were allowed to do full range of motions of surrounding joints. No topical scar therapy was given postoperatively.
During the same period, we have also used HA in 10 adult patients with soft tissue defects secondary to trauma and tumoral excisions. In this subgroup of patients, the acute burn and traumatic wounds were scrubbed with chlorhexidine and then treated with silver sulphadiazine for the next fex days. In all patients, radical debridement was performed within 72h after hospitalisation. Table 2 shows demographic data of this group. Early evaluation of the wounds took place on day 5 and 14 determining the existence of hematoma and infection under the matrix. The same treatment protocol was applied and the patients were followed at least 6 months, hereby the assesment on the VSS were determined by an experienced investigator.
Table 2.
Demographics in group 2 patients
| Patient/age/sex | Follow-up | localisation | Etiology |
|---|---|---|---|
| OS/34 years/male | 3 months | Left forearm | Traumatic amputation |
| AP/20 years/male | 6 months | Left pretibial | Trauma |
| MK/39 years /male | 6 months | Left pretibial | Trauma |
| RN/19 years/ male | 1 year | Left thumb | Traumatic amputation |
| AY/19 years/male | 6 months | Right palm | 3.degree burn |
| EE/18 years/female | 3 months | Right thigh | Postexcisional |
| YE/27 years/male | 3 months | Left forearm | Postexcisional |
Scar evaluation
Vancouver scar scale (VSS) was used to make comparision between preoperative and postoperative scar evaluations (Figures 1, 2 and 3). Preoperative scar assesment was done just before surgical intervention. Postoperative scar evaluations were done between the period of postoperative third and 12th mounth.
Figure 1.
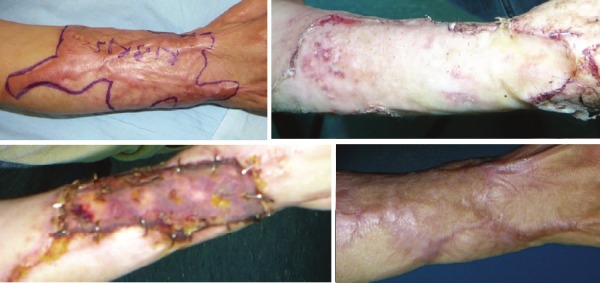
Upper left: a postburn HS on dorsal side of right forearm. Upper right: hyalomatrix application after excision of the marked scar area. Below left:early after skin grafting to the defect. Below right; marked improvement in VSS scores except pigmentation.
Figure 2.
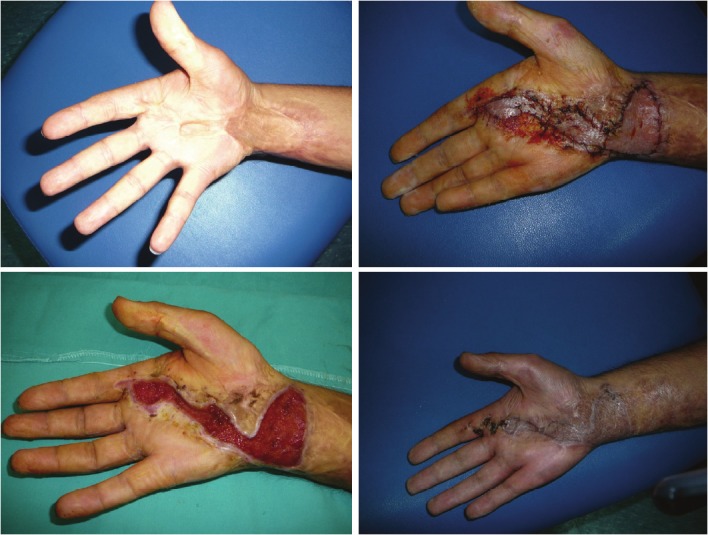
Upper left: a flexion contracture on the right wrist of young male who had two previous skin graft operation.Upper right: early after skin grafting. Below left: 21st day of hyalomatrix application. Below right: at 6th postoperativemonth, no limitation of the adjacent joints is seen.
Figure 3.
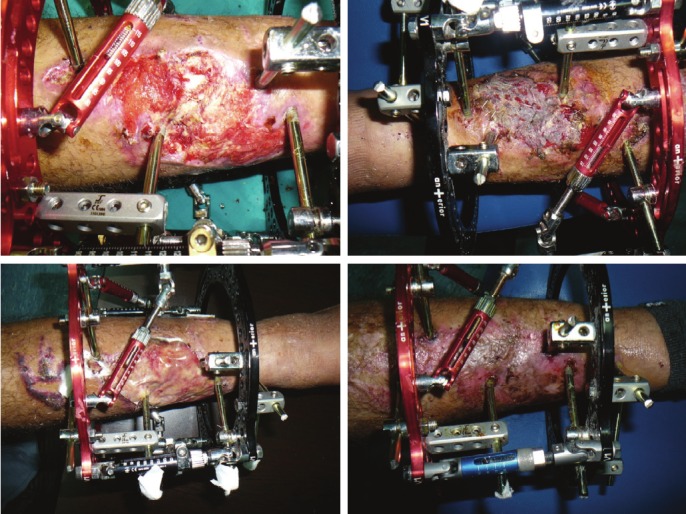
Upper left: Apretibial posttraumatic wound exposing bare bone. Upper right: early after skin grating. Belowleft: hyalomatrix application to the wound. Below right: late result of the treatment.
Histopathologic evaluation
Skin biopsies were obtained either preoperatively or postoperatively, and fixed in 10% neutral buffered formalin solution and then embedded in paraffin. Serial sections were cut using a microtome at a thickness of 4 mm and the sections were stained with hematoxylen eosine (HE). The histopathologic sections were examined by an experienced pathologist for the presence of collagenisation and vascularisation under a microscope (Olympus BX50F4, Tokyo, Japan) and photographed (Figures 4-5). Three representative photomicrographs were obtained from each specimen section. All slides were analysed by a trained observer blinded to whom the specimen had been taken from. The following scale was used to determine the severity of collagenisation and vascularisation; Poor-1, Moderate-2, Severe-3. Mean scores of preoperative skin biopsies were compared with the postoperative scores.
Figure 4.
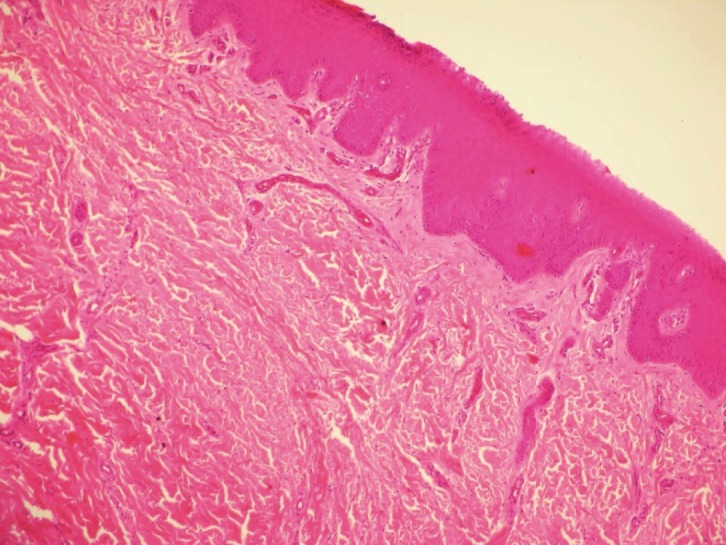
A preoperative skin biopsy showing markedcollagenisation and decreased vascularisation (HEmagnification x200).
Figure 5.
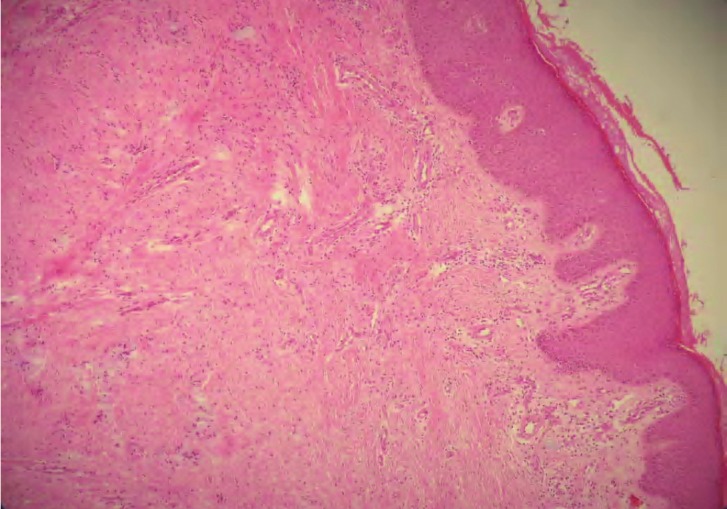
A postoperative skin biopsy shows marked vascularisation and decreased collagenisation with perivascular lymphocyte infiltration (HE, magnificationx 200).
Statistical analysis
All data were expressed as mean ± standart deviation, percentages or mean (confidence interval CI). Preoperative and postoperative VSS scores were analysed using Wilcoxon Signed rank test. Histhopathologic scores obtained were compared with Paired student T test. The level of sifnificance was set at α=0,05.
Results
Demographics and other parameters
Mean age in group 1 was 25.1 ± 10.2 and follow-up time was 6.3 ± 3.6 months. Mean age and follow-up time were 26.4 ± 8 years and 5.5 ± 3.2 months respectively. The data is shown in Tables 1, 2 and 3. In group 2, these parameters were 26.4 ± 8 years and 5.5 ± 3.2 months. There was no occurrence of HS or K development in the follow-up period in any patient (0/7, 0%).
Table 3.
Descriptive data in group 2
| Parameter | Mean or percentage(%) |
|---|---|
| Age | 26.4 ± 8 years |
| HS or K occurence rate | 0/7 (%0) |
| Follow-up | 5.5 ± 3.2 months |
VSS scores
Table 4 shows the VSS scores in group 1. Preoperative mean VSS score was 10.7 ± 1.16 and postoperative was 6.2 ± 0.91. The difference between was significant (p<0.005). All subparameters (pliability, height, etc.) of VSS showed an improved score except pigmentation. Pigmentation never showed an favorable score in posttreatment period even it worsened.
Table 4.
The data in group 1 patients and comparision of VSS scores
| Parameter | Mean ± standart deviation |
|---|---|
| Age | 25.2 ± 10.2 years |
| Follow-up | 6.5 ± 3.6 months |
| Preoperative VSS score | 10.7 ± 1.16* |
| Postoperative VSS score | 6.2 ± 0.91* |
P<0.005, Wilcoxon Signed Rank Test.
Pathologic results
7 of 10 patients in group 1 gave permission of taking skin biopsy at the third postoperative month. Table 5 shows mean scores of preop and postop skin biopsies. There were significantly higher dermal collagenisation in preop biopsies than postop one (p<0.0001). Postop biopsies showed significantly higher vascularization in both reticular and papillary dermis than preop biopsies p<0.0001). Another consistent histhopathologic finding was perivascular lymphocyte infiltration in all postop skin biopsies.
Table 5.
Histhopathologic scores before treatment and three months after the HA application
| Parameter | Preoperative | Postoperative |
|---|---|---|
| Collagenisation | 2.8 ± 0.31 | 1.14 ± 0.3* |
| Vascularisation | 1.2 ± 0.43 | 3.7 ± 0.4** |
Scoring system: for collagenisation zero=no collagen,
=mild collagen formation,
2=moderate collagen formation, 3=heavy collagen formation. For vascularisation, zero=no vascularity, 1=mild vascularisation, 2=moderate vascularisation,
=heavy vascularisation.
P<0.001,
p<0.0001 (Paired samples t-test).
Discussion
Excessive scarring can affect a patient’s quality of life, both physically and physcologically, so there is a clear need for treatment. HS has a rapid growth phase for up to 6 months and then gradually regresses over a period of a few years, eventually leading to flat scars with no further symptoms. K in contrast may develop up to several years after minor injuries. Histologically both contain an abundance of dermal collagen. HS contains primarily type III collagen oriented parellel to the epidermal surface with abundant nodules containing myofibroblasts, large extracellular filaments and plentiful acidic mucopolisaccarides.
Hyalomatirx PA is spesifically intented for the treatment of deep burns and full-thickness wounds. It provides excellent wound preparation to support the implantation of skin grafts. The wound contact layer is constituted by an absorbent, biodegradable, nonwoven pad, with fibers entirely composed of HYAFF (Fidia), a benzyl ester of HA. The three-dimensional scaffold is promptly colonized by fibroblasts and ECM components, favoring an ordered reconstruction of the dermal tissue. After application of a dermal layer, the epidermal layer can be reconstructed by adding a (meshed) STG or cultured keratinocytes. The internal layer acts a biological matrix that acts by modifying extracellular matrix that plays an important role in the process of tissue regeneration [8]. Esterified HA obtains a stable cross-linked matrix which will not liquefy and will postpone degradation [9]. Some of the degradation products of it modulate wound healing and are proangiogenetic [10]. Caravaggi et al published a multicenter study involving 70 centers and 262 elderly patients with vascular, diabetic and traumatic ulcers. They reported that re-epithelization in 83% in a median time of 16 days only using HPA [11].
Gravente et al used Hyalomatrix PA in deep partial burns and found that is useful for reducing postburn HS development in deep partial burns [6]. This matrix was evaluated by several trials by Perrot and Gravante [12]. We observed an improved VSS scale score at the follow-up period when comparing preoperative scores. In other words, total patient score of substituted scars was lower than preoperative score (p<0,001). Furthermore, we have not seen any HS or keloid development in posttraumatic or postexcisional defect group. Our subjective results are comparable to the results of other dermal substitutes [13,14].
In the early inflammation phase HA accumulates in the wound tissue and interacting with CD44 induces proinflammatory cytokines and enhances cell infiltration [15]. What mechanisms are reasonsible for the reduced histopathological collagen deposion as seen in this study is not clear, but some of them may be related to the antioxidant properties of HA which prevent oxygen free radical damage of granulation tissue. During wound healing spesific regulation of HA synthesis and catabolism is responsible for various cellular responses by interacting with HA receptors [16].
Before discussion of the limitations of this study, no randomisation was used for choosing the treatment of the wound pair. For the first time, an histopathologic evaluation of Hyalomatrix skin substitution in man was performed to investigate scar assesment in the present study. It was remarkable that subjective scores were correlated with the pathologic findings. However it should be noted that the observations of this study could not be blinded, therefore a bias can not be excluded. The other limitation of the study is that the histopathologic assesment was not based on advanced evaluation. But simple hematoxylen eosine examinations of the preoperative scar and postoperative substituted skin tissues showed a definitive difference between pretreatment and posttreatment of the scar areas. There were both decreased collagenisation and increased vascularisation in skin biopsies after HA treatment (P<0.001 and p<0.0001 respectively). Furtherly this histopathologic findings were supported with the clinical evaluations, as postoperative VSS scores of treated areas showed an improved VSS scores than preoperative scores (p<0.005). We can not make any definitive claims about the effectiveness of the dressing, as this has not been adequately assesed in prospective observational study. More studies on tissue engineering and skin substitution may result in more evidence and support for its long-term clinical effectiveness.
References
- 1.Brusselaers N, Lafaire C, Ortiz S, Jacquemin D, Monstrey S. The consensus of the surgical treatment of burn injuries in Belgium. Acta Chir Belg. 2008;108:645–650. doi: 10.1080/00015458.2008.11680309. [DOI] [PubMed] [Google Scholar]
- 2.Haslik W, Kamolz LP, Nathschlager G, Andel H, Meissl G, Frey M. First experiences with the collagen-elastin matrix Matriderm as a dermal substitute in severe burn injuries of the hand. Burns. 2007;33:364–368. doi: 10.1016/j.burns.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Branski LK, Herndon DN, Perrera C, Mlcak RP, Celis MM, Lee JO, Sanford AP, Norbury WB, Zhang XJ, Jeschke MG. Longitidunal assesment of integra in a primary burn management: a randomised pediatric clinical trial. Crit Care med. 2007;35:2615–2623. doi: 10.1097/01.CCM.0000285991.36698.E2. [DOI] [PubMed] [Google Scholar]
- 4.Russel H, Guyalan E, German G, Öhlbauer M. The use of matriderm in early excision and smultaneous autologous skin graft in burns: a pilot study. Burns. 2008;34:93–94. doi: 10.1016/j.burns.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Altman RD. Intra-articular sodium hyaluronate in osteoarthritis of the knee. Seminars in Arthritis and Rheumatism. 2000;30:11–18. doi: 10.1053/sarh.2000.0248. [DOI] [PubMed] [Google Scholar]
- 6.Gravante G, Delogu D, Giordan N, Morano G, Montone A, Esposito G. The Use of Hyalomatrix PA in the treatment of deep partial-thickness burns. J Burn Care Res. 2007;28:269–274. doi: 10.1097/BCR.0B013E318031A236. [DOI] [PubMed] [Google Scholar]
- 7.Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing current biology. World J Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 8.Myers SR, Portha VN, Soranzo C, Price RD, Nausaria HA. Hyalomatrix a temporary epidermal barrier hyaluronan and neodermis induction system prior keratinocyte stem cell therapy. Tissue Eng. 2007;13:2733–2741. doi: 10.1089/ten.2007.0109. [DOI] [PubMed] [Google Scholar]
- 9.Price RD, Berry MG, Navsaria HA. Hyaluronic acid: the scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007;60:1110–1119. doi: 10.1016/j.bjps.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Sattar A, Rooney P, Kumar S, Pye D, West DC, Scott I, Ledger P. Application of angiogenetic oligosaccharides of hyaluronan increases blood vessel numbers in rat skin. J Invest Dermatol. 1994;103:576–579. doi: 10.1111/1523-1747.ep12396880. [DOI] [PubMed] [Google Scholar]
- 11.Caravaggi C, Grigoletto F, Scuderi N. Wound bed preparation with dermal substitute (Hyalomatrix PA) fascilites re-epithelization and healing: Results of multicenter, prospective, observational study on chronic complex ulcers. Wounds. 2011;23:228–235. [PubMed] [Google Scholar]
- 12.Perrot P, Dellière V, Brancati A, Duteille F. Hyalomatrix PA(®) in skin substitutes. About 10 cases. Ann Chir Plast Esthet. 2011;56:107–111. doi: 10.1016/j.anplas.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Gravante G, Sorge R, Merone A, Tamisani AM, Di Lonardo A, Scalise A, Doneddu G, Melandri D, Stracuzzi G, Onesti MG, Cerulli P, Pinn R, Esposito G. Hyalomatrix PA in burn care practice: results from a national retrospective survey, 2005 to 2006. Ann Plast Surg. 2010;64:69–79. doi: 10.1097/SAP.0b013e31819b3d59. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer E, Queruel P, Saliner L, Palmier B, Quinas JF. Dermal regeneration template for deep hand burns Clinical utility for both early grafting and reconstructive surgery. Br J Plast Surg. 2003;56:764–774. doi: 10.1016/s0007-1226(03)00366-7. [DOI] [PubMed] [Google Scholar]
- 15.Trabachi E, Pallotta S, Morini M, Corsi F, Franceshini R, Casiraghi A, Pravettoni A, Foschi D, Minghetti P. Low molecular weight hyaluronic acid prevents oxygen free radical damage to granulation tissue during wound healing. Int J Tissue React. 2002;24:65–71. [PubMed] [Google Scholar]
- 16.Chen WY, Abatangelo G. Function of hyaluronan in wound repair. Wound rep Reg. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]


