Abstract
Background
Many plants with antidiabetic properties probably act in part through their content of fibre, vitamins, bioactive or mineral content
Objectives
This study investigated the mineral, proximate, phytochemical compositions and hypoglycaemic effect of Commelina africana and Ageratum conyzoides extracts in diabetic rats, and the likely relationship between this property and the mineral, proximate and phytochemical compositions of the plants.
Methods
The plants were subjected to mineral, proximate composition and phytochemical analysis. Attempt was made to see (if any) the relationship between the hypoglycaemic effect and the mineral, proximate compositions and phytochemistry of the plants. Alloxan-induced diabetic animals were administered 500mg/kg body weight aqueous extracts of the plants and glibenclamide as the reference hypoglycaemic agent.
Results
Aqueous extract of Ageratum conyzoides reduced fasting blood glucose of experimental animals by 39.1% while Commelina africana reduced the same by 78.0%. Alkaloids, cardenolides, saponins, and tannins were detected in both plants. Anthraquinones was absent in C. africana but a trace of it was detected in A. conyzoides. The hypoglycaemic effect of Commelina africana was comparable with the reference hypoglycaemic agent. Ageratum conyzoides showed comparably weaker hypoglycaemic effect than exhibited by reference hypoglycaemic agent. Comparatively, Commelina africana had higher mineral concentrations (except Na) than Ageratum conyzoides.
Conclusions
Plants' extracts minerals (magnesium, potassium and iron) and bioactive components (alkaloids and cardenolides) seemingly enhanced their hypoglycaemic effect. Furthermore, these minerals, alkaloids and cardenolides could be helpful in ameliorating complications of diabetes like hypertension and cardiovascular disease.
Keywords: Commelina africana, Ageratum conyzoides, diabetes
Introduction
Diabetes is a chronic disorder of glucose intolerance. This disease is alarming and has been described as a major cause of disability and death.1 It is characterised by high blood glucose level and glycosuria resulting from dysfunction of pancreatic â-cells and insulin resistance. Mineral deficiencies are common in diabetes and can exacerbate insulin resistance. Several of these minerals are co-factors for signalling intermediaries of insulin action and key enzymes of glucose metabolism2.
The global prevalence rate of Diabetes mellitus places the disease at the top of the chart as one of the most frequent of endocrine diseases.3
Its increased prevalence has been noticeably highest in Asia and Africa probably due to the adoption of a Western dietary lifestyle.4
All forms of diabetes have been treatable since insulin became medically available but a cure is difficult. Currently, diabetes therapy is based on the use of oral hypoglycaemic agents (sulfonylureas, and biguanides) insulin, plant supplements, diet measures and exercises .5 Other current medications include vitamins and minerals. Mineral supplements can benefit patients with mineral deficiencies, as demonstrated with magnesium and zinc .2 A lifelong treatment of diabetes has been shown to entail an enormous financial outlay for a patient. The natural dietary adjuncts have been found to serve as functional foods through which patients can gain added benefits to the management of the disease6, 7
Diet is also known to be the foundation of diabetic control, and the dietary recommendations for diabetic patients are entirely consistent with a normal healthy balanced diet. 7 Table salt, refined sugars and foods rich in cholesterol have to be minimised while ensuring adequate vitamins and mineral.
Several plants of diverse origins have been exploited by trial and error over many generations for therapeutic purposes.8, 9 In Africa and in most of the developing countries, plants' properties are empirically appreciated.9 The adverse effects of chemical drugs, their increasing costs and greater public access to information on traditional medicine have also led to an increase in interest in alternative treatments.10 Also, many urban populations have turned to herbal treatments. The reason is that traditional medicine is a medicine of proximity, less constraining and non- expensive.11 In the light of this, The World Health Organization Expert Committee on diabetes has recommended that traditional methods of treatment for diabetes should be sought vigorously.12
Although an orally active botanical substitute for insulin is unlikely, phytochemicals may stimulate insulin biosynthesis and promote insulin action.13Many plants with antidiabetic properties probably act in part through their content of fibre, vitamins or mineral content. To this end, plants rich in minerals have been shown to enhance glycaemic control in diabetic patients.2, 14
Ageratum conyzoides is an herbaceous plant used in traditional medicine in several countries of the world. The use of this herb as a bacteriocide, antidysentric, antipyretic, antirheumatic and antibiotic has been reported.5 The advantageous effect of the ethanolic leaf extract on hematological indices of rats has also been documented. Commelina africana is a perennial herb used traditionally for irregular menstrual flow.
The objective of this work was to investigate the anti-diabetic effect of fresh leaves of Commelina africana, and Ageratum conyzoides in diabetic rats with a view to evaluating their beneficial hypoglycaemic effect and to show the relationship between this property and the plants' mineral/ chemical compositions.
Methods
Experimental animals
Wistar albino rats, 110–210g body weight, were obtained from Animal House, University of Ibadan, Nigeria. They were all fed pellets rat feed (Ladokun Feeds Ibadan, Nigeria). All the animals were acclimatised 7 days before the commencement of the experiment and housed under natural cycles of night and day in the animal house, Lead City University.
Collection and extraction of plant leaves
Fresh leaves of Commelina africana and Ageratum conyzoides were collected from the garden of the Department of Forestry, University of Ibadan. Confirmatory identification of the plants was done at the Forestry Research Institute of Nigeria (FRIN), Ibadan, Nigeria. Some of the leaves were air dried, ground to powdery form and stored pending use or analysis. Some fresh leaves were weighed and rinsed with distilled water to remove extraneous materials. Ten (10 g) gram weight of the leaves was then pulverized and mixed with 100ml distilled and sieved using filter paper. The extracts so obtained were stored in the refrigerator after use. Fresh aqueous extracts were prepared at three days interval.
Diabetes induction and drug administration
Diabetes was induced in rats by a single intraperitoneal administration of 5% alloxan at 125mg/kg body weight. Rats with blood glucose level of 150mg/dl and above were considered diabetic. After confirmation of induction using electronic Glucometer, 48 hours after administration of alloxan, diabetic rats were randomly distributed into groups of five (5) animals per group. Group 1 was non-diabetic rats administered distilled water. Group 2 was diabetic untreated rats. Group 3 was diabetic rats administered reference hypoglycaemic agent (10mg/kg Glibenclamide). Group 4 was diabetic rats administered 500mg/kg body weight aqueous extract of Ageratum conyzoides. Group 5 was diabetic rats administered 500mg/kg body weight aqueous extract of Commelina africana.
Animals were deprived of food 12 hours to determine their Fasting Blood Glucose (FBG) levels before reintroduction of food to the animals. The initial blood glucose (Day 0) was taken as the baseline. Drug and extracts were administered once daily for 7 days. On each day, FBG was obtained before administration of drug and extracts.
Analytical procedures
Proximate and mineral compositions of the plant were carried out according to the Official Methods of Analysis of Association of Official Analytical Chemist.15
Phytochemical screening
The Phytochemical screening of the plants was carried out by the standard methods described by Harbone16 and Trease & Evans.17 Bioactive constituents assayed included saponins, tannins, total alkaloids, cardiac glycosides, and anthraquinones.
Statistical analysis
All assays were carried out in duplicates except otherwise stated. Values were expressed as mean ± standard deviation.
Results
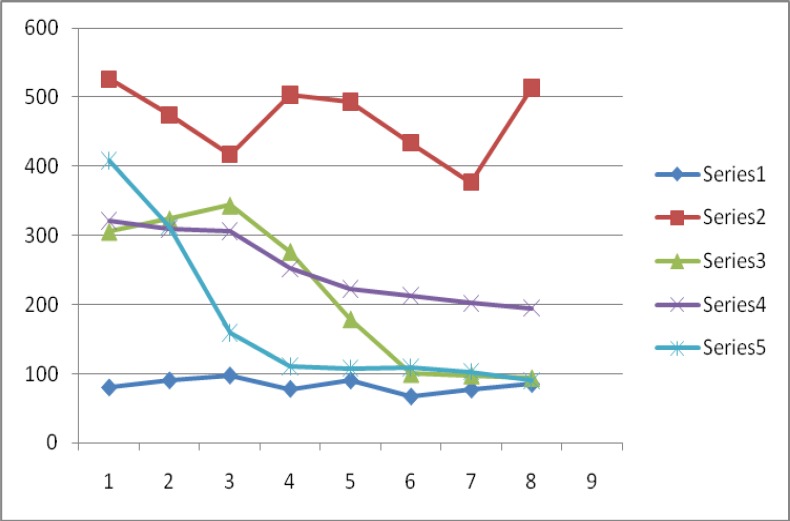
Figure 1 shows the effect of glibenclamide and plant extracts on fasting blood glucose. Figure 2 shows the percentage reduction in fasting blood glucose of the rats under study. Non- diabetic, normoglycaemic rats showed normal blood glucose within the range 80.7mg/dl–97.6mg/dl. The diabetic untreated rats had hyperglycaemia throughout the experimental period with fasting blood glucose of 526.7mg/dl on zero day to 513mg/dl on the last day of the experiment (day 7). The reference hypoglycaemic agent, glibenclamide, reduced the blood glucose from an initial 306.3mg/dl (hyperglycaemia) to 94.3mg/dl (normoglycaemia). This was a 69.2% total reduction over a period of 7 days. Aqueous extract of Ageratum conyzoides reduced fasting blood glucose of experimental animals from 309.6mg/dl (hyperglycaemia) to 195.8mg/dl (hyperglycaemia), a 39.1% total reduction in blood glucose. Extract of Commelina africana reduced fasting blood glucose from 408.5mg/dl (hyperglycaemia) to 90.0 (normoglycaemia), a 78.0% reduction.
Figure 1.
Fasting blood glucose levels of diabetic rats following administration of Glibenclamide, Ageratum conyzoides and Commelina Africana
Series 1- non-diabetic rats, Series 2- diabetic untreated rats, Series 3- diabetic rats administered glibenclamide, Series 4- diabetic rats administered Ageratum conyzoides, Series 5- diabetic rats administered Commelina Africana
Figure 2.
Percentage difference between day 7 and day 0 of rats
non-diabetic rats, 2- diabetic untreated rats, 3- diabetic rats administered glibenclamide, 4- diabetic rats administered Ageratum conyzoides, 5- diabetic rats administered Commelina Africana
Table 1 shows the mineral composition of the plants under study. Comparatively, Commelina africana had higher mineral composition (except Na) compared with Ageratum conyzoides.
Table 1.
Mineral composition of Commelina africana and Ageratum conyzoides
| Minerals |
Commelina africana |
Ageratum conyzoides |
| P (g/kg) | 15.52 | 4.49 |
| K (g/kg) | 30.9 | 11.91 |
| Na (g/kg) | 8.87 | 11.71 |
| Mg (g/kg) | 2.41 | 0.82 |
| Ca (g/kg) | 48.60 | 2.21 |
| Fe (mg/kg) | 1500.00 | 161.00 |
| Zn (mg/kg) | 142.70 | 9.85 |
| Mn (mg/kg) | 33.00 | 0.50 |
| Cu (mg/kg) | 15.70 | 6.90 |
Table 2 shows the proximate composition of Commelina africana and Ageratum conyzoides. The two plants showed similar proximate composition. For example crude fat for C. Africana was 4.18% and 5.67% for Ageratum conyzoides. Crude protein was 17.86 for Commelina africana and 15.67 for Ageratum conyzoides.
Table 2.
Proximate composition of Commelina africana and Ageratum conyzoides
| % Composition |
Commelina africana |
Ageratum conyzoides |
| Crude protein | 17.86 | 15.67 |
| Crude fat | 4.18 | 5.67 |
| Crude fibre | 19.21 | 18.24 |
| Total ash | 13.87 | 16.72 |
| Moisture content | 10.53 | 10.33 |
| Carbohydrate | 34.35 | 33.37 |
| Fat matter | 89.47 | 89.67 |
Table 3 shows the phytochemical composition of Commelina africana and Ageratum conyzoides. Alkaloids, cardenolides, saponins, and tannins were detected in both plants. Anthraquinones were however completely absent in C. africana, with only their traces detected in A. conyzoides.
Table 3.
Phytochemistry of Commelina africana and Ageratum conyzoides
| Plant |
Commelina africana |
Ageratum conyzoides |
| Alkaloids | + | + |
| Cardenolides | + | + |
| Anthraquinones | − | ± |
| Saponins | + | + |
| Tannins | + | + |
Discussion
The persistent hyperglycaemia in alloxan-induced diabetic untreated rats throughout the period of the experiment was as a result of beta cells destruction in agreement with a previous report.18 Administration of 500mg/kg body of extracts of Commelina africana and Ageratum conyzoides caused 78.0% and 39.1% fasting blood glucose level reduction respectively over a period of 7 days. While Commelina africana extract caused a remarkable hypoglycaemic effect, Ageratum conyzoides's extract still manifested a mild hyperglycaemia. The hypoglycaemic effect of Commelina africana was comparable with the reference hypoglycaemic agent (glibenclamide). Ageratum conyzoides showed weak hypoglycaemic effect incomparable with that of the reference hypoglycaemic agent. The hypoglycaemic potential of A. conyzoides may be enhanced by combination with other oral hypoglycaemic agent(s) especially of plant source, consistent with the report of Sofowora.19
A number of mechanisms could be adduced for the hypoglycaemic effect of Commelina africana and Ageratum conyzoides. In support of the present findings by other studies it could be postulated that apart from having inhibitory effect on glucose absorption, it is probable that other mechanisms such as direct stimulation of glycolysis in peripheral tissues, facilitation of glucose entry into peripheral cells, reduced hepatic gluconeogenesis and reduction of plasma glucagon levels may be in operation.20, 21
Commelina Africana and Ageratum conyzoides have both been known to contain alkaloids, some of which may be associated with the improvement in the symptoms of diabetes mellitus.22 The causes and sites of intervention in biochemical process in diabetes are diverse .23 It is likely that the interplay of diversity of action could be applicable in the observed hypoglycaemic effect of the leaf extracts of Commelina africana and Ageratum conyzoides containing several biologically active components.
Commelina africana showed higher mineral composition than A. conyzoides. Mineral composition of Commelina africana showed a higher concentration of potassium (30.59g/kg) than sodium (8.87g/kg). This could be an enhancement factor in favour of the higher hypoglycaemic effect of the extract and possible prospect in the management of high blood pressure, a complication of diabetes. Although sodium and chloride are important, potassium is the most important dietary electrolyte. Potassium helps to maintain water balance and distribution, blood pressure, acid-base balance, muscle and cell nerve function, heart function, kidney and adrenal function.24 Diet low in potassium and high in sodium is associated with high blood pressure. Ageratum conyzoides contained lower concentration of potassium and iron (11.91g/kg, 161mg/kg respectively) and higher sodium (11.71g/kg) compared with Commelina africana. This might be responsible for the relatively lower hypoglycaemic effect. Also, the higher sodium content may not make the extract a comparatively better candidate for ameliorating high blood pressure, a complication of diabetes.24, 25
Commelina africana extract was observed to be rich in Iron which may increase Packed Cell Volume (PCV) and boost the immune system, during diabetic state.25, 26 It can be hypothesised that Ageratum conyzoides is a good source of nutrient but not an ideal plant that can elicit a desired hypoglycaemic effect when administered singly. Zinc has been shown to be involved in blood sugar control mechanism and protect against diabetes.27 This mineral was found to be abundant in Commelina Africana, further laying credence to its hypoglycaemic activity. The high content of manganese in Commelina africana may enhance the observed beneficial effect in regulating blood sugar level. Its deficiency symptoms include high blood sugar levels, joint pain and poor memory.25
Both plant extracts contained high magnesium concentration which has been reported to be helpful in fighting heart disease, stroke and assist in cell repair.25 The high fibre contents of Ageratum conyzoides and Commelina africana may serve as stool bulking agent for prevention of constipation while their high crude protein content suggests their contribution to body cell repairs and growth.28
Both plants extracts with reasonable composition of alkaloids, especially cardiac glycosides, may be helpful in alleviating hyperglycaemia, coronary heart disorder and other complications of diabetes.17, 22, 29
Conclusion
A combination of natural fibre, complex carbohydrate, antioxidants and minerals is beneficial in diabetics.6 Some of these minerals are co-factors for signalling intermediaries of insulin action and key enzymes of glucose metabolism.2 The plants under study were observed to be rich in these. An added value is the observed hypoglycaemic effects of the plants. This is in line with findings in plant like Atriplex halimus2. Further work would be necessary to subject the plant extracts to column chromatography and other processes to obtain the structures of the most active component (s). This would, among others, be helpful in elucidating their mechanisms of action.
References
- 1.Kameswara R, Giri R, Kesavulu MM, Apparao C. Herbal medicine: In the management of diabetes mellitus. Manphar Vaidhya Patrica. 1997;1:33–35. [Google Scholar]
- 2.Day C. Hypoglycaemic compounds from plants. In: Bailey CJ, Flatt PR, editors. In New Antidiabetic Drugs. Smith-Gordon, London: 1990. pp. 267–278. [Google Scholar]
- 3.Grover JK, Yadav S, Vats V. Medicinal plants of India with antidiabetic potential. Journal of Ethnopharmacology. 2003;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetic Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Menut C, Sharma S, Luthra C. Aromatic plants of tropical central Africa, Part X Chemical composition of essential oils of Ageratum houstonianum Mill. and Ageratum conyzoides L. from Cameroon. Flav Fragr Journal. 1993;8(1):1–4. [Google Scholar]
- 6.Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional dietary adjuncts for the treatment of diabetes mellitus. Proceedings of the Nutrition Society. 1991;50:641–651. doi: 10.1079/pns19910077. [DOI] [PubMed] [Google Scholar]
- 7.Day C. Invited commentary: Traditional plant treatments for diabetes mellitus: pharmaceutical foods. British Journal of Nutrition. 1998;80:5–6. doi: 10.1017/s0007114598001718. [DOI] [PubMed] [Google Scholar]
- 8.Iwu MM. African Ethnomedicine. Enugu, Nigeria: SNAAP Press; 1986. pp. 20–25. [Google Scholar]
- 9.Sofowora A. Spectrum Books LTD. Sunshine House 1, Emmanuel Alayande Street, P.M.B 5612, Ibadan, Nigeria: 1996. Medicinal plants and Traditional Medicine in Africa; pp. 15–72. [Google Scholar]
- 10.World Health Organisation, author. WHO Traditional Medicine Strategy 2002–2005. Geneva: World Health Organization; 2002. [Google Scholar]
- 11.Shukla R, Sharma SB, Puri D, Pabhu K M, Murthy P S. Medicinal plants for treatment of diabetes mellitus. Indian Journal of Clinical Biochemistry (Suppl.) 2000;15:169–177. doi: 10.1007/BF02867556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation, author. Expert committee: Diabetes Mellitus 2nd rep. Geneva: World Health Organization; 1998. 180 (Tech Rep ser 646) [Google Scholar]
- 13.Bailey CC, Bailey OT. The production of diabetes mellitus in rabbits using Alloxan. Journal of American Medicine. 1943;21:1165. [Google Scholar]
- 14.Day C. Hypoglycaemic plant compounds. Practical Diabetes. 1995;12:269–271. [Google Scholar]
- 15.Official Method of Analysis. Washington DC: Association of Analytical Chemists (AOAC); 2004. [Google Scholar]
- 16.Harbone JB. Phytochemical Methods. London: Chapman and Hall; 1993. [Google Scholar]
- 17.Trease G, Evans WC. Trease & Evans' Pharmacognosy. 4th ed. USA: WB Sounders; 2002. pp. 243–283. [Google Scholar]
- 18.Prince PSM, Menon VP. Hypoglycemic activity of Syzigium cumini seeds: effect of lipid peroxidation in alloxan diabetic rats. Journal of Ethnopharmacology. 1998;61:1–7. doi: 10.1016/s0378-8741(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Sofowora A. Medicinal Plants and Traditional Medicines in Africa. New York: Chichester John, Wiley & Sons; 1993. pp. 97–145. [Google Scholar]
- 20.Aderibigbe AO, Emudianughe TS, Lawal BAS. Anti-hyperglycaemic effects of Mangifera indica in rats. Phytotherapy. 1998;13:504–507. doi: 10.1002/(sici)1099-1573(199909)13:6<504::aid-ptr533>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Emudianughe TS, Aderibigbe AO. The hypoglycaemic effect of Telfaria occidentalis aqueous leaf extract in rats. West African Journal of Pharmacology. 2002;18(1&2):14–16. [Google Scholar]
- 22.Grupta SA, Seth CB. Effect of Mordica chanrantia on glucose tolerance on albino rats. Indian Journal of physiology & pharmacology. 1962;7:240–244. [Google Scholar]
- 23.Akah PA, Okafor CI. Blood sugar lowering effect of Vernonia amydalina Del, in an experimental rabbit model. Phytotherapy Res. 1992;16:171–173. [Google Scholar]
- 24.Murray M, Joseph P. Textbook of Natural Medicine. 3rd ed. New York: Churchill Livingstone; 2008. [Google Scholar]
- 25.Kurtzweil P. Nutrition Facts to Help Consumers Eat Smart. FDA Consumer; 1993. [Google Scholar]
- 26.Ahmed MS, Reid E, Khardori N. Respiratory infections in diabetes: Reviewing the risks and challenges. Journal of Respiratory Diseases. 2008;29(7):10–14. [Google Scholar]
- 27.Fallon SAF, Enig MG. Nourishing Traditions. 2nd ed. Washington DC: New trends publishing; 2001. [Google Scholar]
- 28.Rewers M, Gottlieb P. Immunotherapy for the prevention and treatment of Type 1 diabetes: human trials and a look into the future. Diabetic Care. 2009;32(10):1769–1782. doi: 10.2337/dc09-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratton IM, Adler AI, Neil AW. Association of glycaemia with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 35): prospective observational study. British Medical Journal. 2000;3:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]