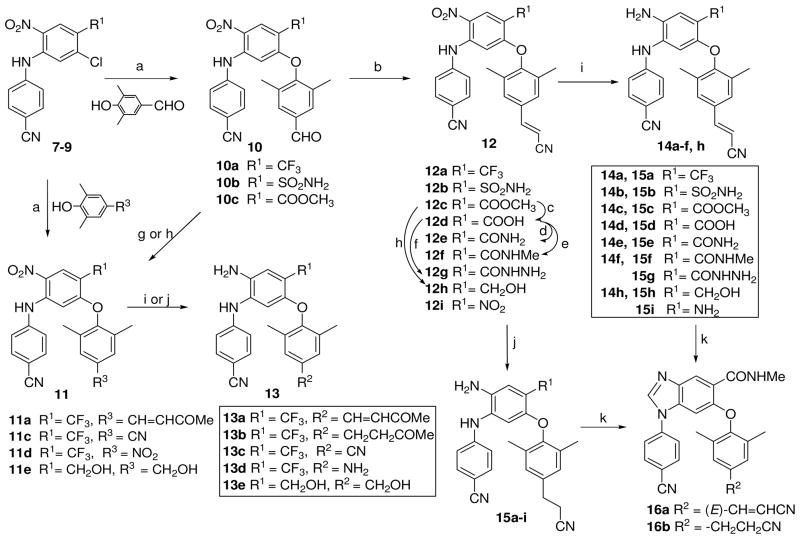
Scheme 2.
a) K2CO3/DMF, 190 °C, MW, 15 min or traditional heating at 130 °C, 5 h; b) (EtO)2P(O)CH2CN, t-BuOK/THF, 0 °C – r.t., 3 h; c) aq NaOH/THF/MeOH, r.t., 4 h; d) (i) SOCl2/CH2Cl2, reflux, 3 h; (ii) NH4OH/THF, 0 °C, 0.5 h; e) (i) SOCl2/CH2Cl2, reflux, 3 h; (ii) NH2CH3 in THF, 0 °C, 0.5 h; f) (i) SOCl2/CH2Cl2, reflux, 3 h; (ii) N2H4·H2O, r.t, 1 h; g) CH3COCH3, MeOH/aq NaOH (10%), 0 °C – rt, 1 h; h) LiBH4, THF/MeOH, 1 h; i) Na2S2O4/NH4OH, THF/H2O (v/v 1:1), r.t.; j) H2/Pd-C, EtOH, 2–4 h; k) CH(OEt)3, HCl (1N), C2H5OC2H5/DMF, r.t. 3 h.