Table 3.
Antiviral activity against wild-type and mutated viral strains of selected active DAANs
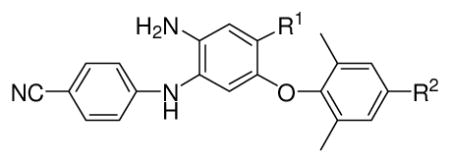
| ||||||
|---|---|---|---|---|---|---|
| R1 | R2 | EC50 (nM)a (Fold Change)b
|
||||
| NL4-3 | RTMDRc | K101Ed | E138Kd | |||
| 14e | CONH2 | CH=CHCN | 0.87 | 1.8 ± 0.5 (2.1) | 8.3 ± 3.3 (9.5) | 9.0 ± 2.6 (10.3) |
| 14h | CH2OH | CH=CHCN | 0.53 | 0.4 ± 0.1 (0.7) | 3.4 ± 1.0 (6.4) | 3.9 ±0.9 (7.4) |
| 15c | COOCH3 | CH2CH2CN | 4.32 | 4.1 ± 0.7 (0.9) | 14 ± 4.3 (3.2) | 7.5 ± 2.7 (1.7) |
| 15f | CONHCH3 | CH2CH2CN | 2.73 | 8.0 ± 4.3 (2.9) | 14 ± 4.8 (5.1) | 10 ± 3.9 (3.7) |
| 15h | CH2OH | CH2CH2CN | 0.39 | 1.8 ± 0.5 (4.6) | 3.4 ± 1.3 (8.7) | 2.9 ± 1.2 (7.4) |
| 3 | NO2 | CH=CHCN | 0.63 | 0.9 ± 0.3 (1.4) | 11 ± 3.6 (17.5) | 12 ± 3.9 (19.0) |
| 2 | 0.52 | 0.5 ± 0.1 (0.9) | 5.7 ± 1.4(11.0) | 5.2 ± 1.6 (10.0) | ||
Experiments performed at least in triplicate and data presented as means± SD.
resistant fold change.
HIV-1 RTMDR (obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), which contains mutations in RT amino acid residues L74, M41L, V106A, and T215Y, is resistant to AZT, ddI, nevrapine, and other noncucleoside RT inhibitors.
mutated amino acids in the HIV-RT non-nucleoside binding pocket (NNBP) confer resistance to 2.
