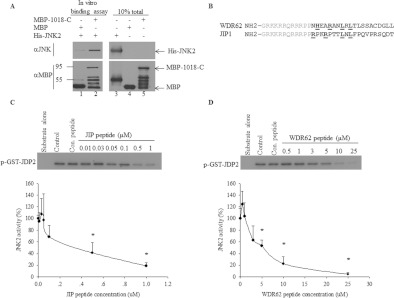
Figure 5. WDR62–JNK association is direct and the WDR62 D domain inhibits JNK activity in vitro.
(A) Bacterially purified MBP, MBP-1018-C and His–JNK2 proteins were mixed as indicated for 2 h at 37°C. Subsequently, protein complexes were attached to amylose resin, washed extensively and then eluted using maltose. Western blot analysis was performed with either anti-JNK (upper panel) or anti-MBP (lower panel) antibodies. (B) Amino acid sequence alignment of WDR62 and JIP1 peptides. The amino acids representing the consensus D domain are underlined. Grey amino acids indicate HIV-Tat sequence that directs cellular import in cells. (C and D) In vitro kinase assay with activated JNK2 pre-incubated with the indicated concentration of JIP (C) and WDR62 (D) peptides. Control HA elution peptide (Con. peptide) was used at 25 μM (lanes 3). Kinase assay was performed in the presence of [γ-32P]ATP and purified GST–JDP2 (5 μg) as substrate. Lanes 1 contained substrate alone without JNK2, and lanes 2 contained JNK2 and substrate without competing peptide. Phosphorylated GST–JDP2 was resolved by SDS/PAGE and detected by autoradiography (upper panels). The extent of GST–JDP2 phosphorylation in the absence of competing peptide is considered as 100% and the extent of JDP2 phosphorylation in the presence of the peptides was calculated relatively (lower panels). Results shown are means±S.E.M. for three independent experiments. *P<0.05.