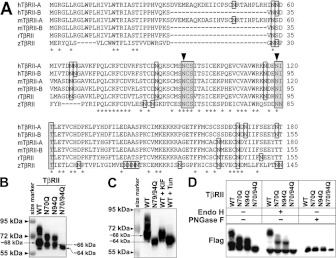
Figure 2. Two conserved asparagine residues are essential for N-linked glycosylation of TβRII.
(A) N-terminal amino acid sequence alignment of human TβRII isoform A (hTβRII-A), human TβRII isoform B (hTβRII-B), mouse TβRII isoform A (mTβRII-A), mouse TβRII isoform B (mTβRII-B), rat TβRII (rTβRII) and zebrafish TβRII (zTβRII). Asparagine residues are boxed. Asn-Xaa-Ser/Thr motifs are shaded. Asterisks indicate conserved residues. Arrowheads indicate conserved asparagine residues in the Asn-Xaa-Ser/Thr motif. (B and C) Western blot analysis showing the protein expression of TβRII in A549 cells. (B) FLAG-tagged TβRII of WT and three dNG mutants N70Q, N94Q and N70/94Q. (C) WT, N70/94Q, KIF-treated WT and Tun-treated WT TβRII. Note that the single mutants or KIF treatment (10 μg/ml for 24 h) reduced N-linked glycosylation levels of TβRII, whereas the double mutant or Tun treatment (1 μg/ml for 12 h) completely block the N-linked glycosylation. (D) Western blots of Endo H- and PNGase F-treated WT and dNG mutants of TβRII. Note that PNGase F treatment removed all N-glycosylated forms of TβRII, whereas Endo H treatment did not remove heavily glycosylated forms of TβRII.