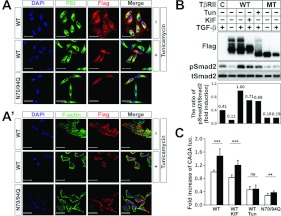
Figure 4. Deglycosylated TβRII blocks TGF-β signalling and cell surface transport of TβRII.
(A and A') Fluorescence micrographs showing the localization of transiently transfected FLAG-tagged WT and N70/94Q TβRII in HeLa cells. Cells were stained with an anti-FLAG antibody (red), PDI (green) (A) or phalloidin (F-actin; green) (A'). Tun treatment was at 1 μg/ml for 12 h. Note that WT TβRII proteins are co-stained with phalloidin at the cell surface. However, Tun-treated WT and N70/94Q TβRIIs accumulate in the perinuclear region, almost merging with PDI. Scale bars, 50 μm. (B) WT and N70/94Q double mutant (MT) TβRII were transiently transfected into A549 cells. At 8 h of transfection, cells transfected with WT TβRII were treated with KIF (10 μg/ml for 24 h) and Tun (1 μg/ml for 12 h), followed by TGF-β1 treatment (5 ng/ml for 30 min). Cell extracts were immunoblotted with anti-FLAG, anti-phospho-Smad2 and anti-Smad2. Band intensities representing phospho-Smad2 and Smad2 expression levels were converted by densitometry using ImageJ software into the ratio of phospho-Smad2 to Smad2. Note that Smad2 phosphorylation is dramatically reduced in the N70/94Q TβRII-transfected cells. (C) WT and N70/94Q TβRII were co-transfected with (CAGA)12-luciferase and β-galatosidase into A549 cells. At 8 h after transfection, cells transfected with WT TβRII were treated with KIF and Tun, followed by TGF-β1 treatment (5 ng/ml for 16 h). Cells were then collected for luciferase (luc.) and β-galatosidase assays. Results represent luciferase activity related to β-galactosidase activity and are means±S.E.M. for experiments in triplicate. Note that the double dNG mutant (N70/94Q) significantly decreased (CAGA)12-luciferase transcriptional activity. ***P<0.001; **P<0.01; ns, not significant.