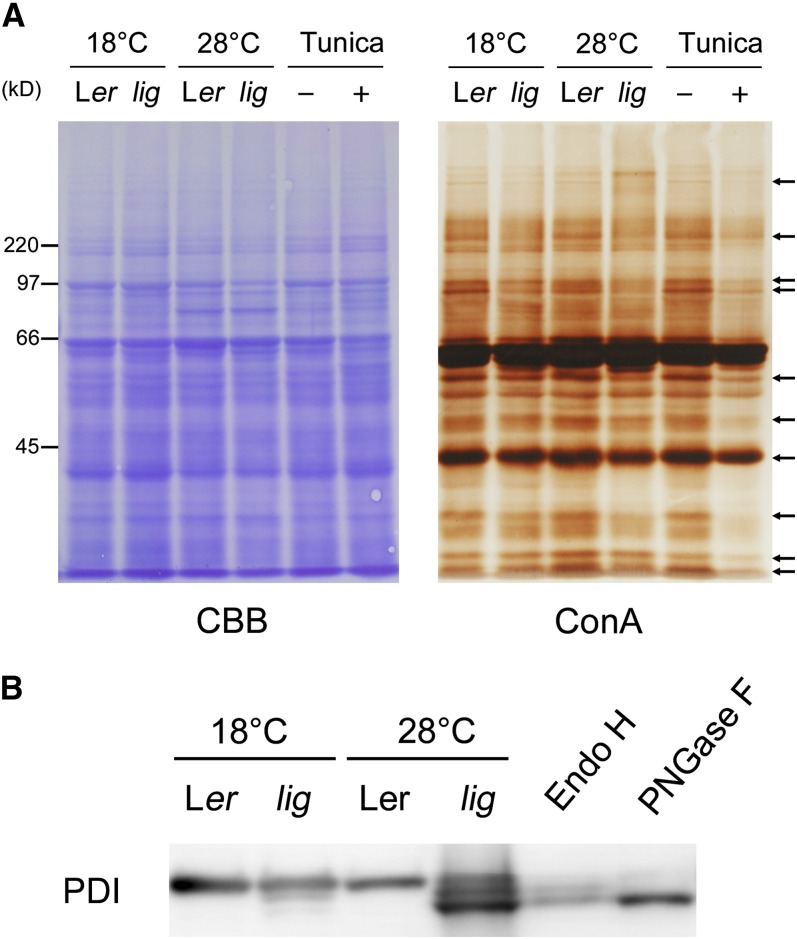
Figure 11.
Effect of the lig Mutation on the Electrophoretic Profiles of N-Glycoproteins.
(A) Proteins were extracted from the roots of seedlings of the wild type (Ler) and lig mutant grown for 5 d at 18°C and then for 3 d at 18 or 28°C, and also from the roots of wild-type seedlings cultured with 100 ng/mL tunicamycin for 3 d after 5 d of tunicamycin-free culture at 18°C. The protein samples were separated by SDS-PAGE and subjected to Coomassie blue (CBB) staining for total protein detection or lectin staining with ConA for N-glycan detection. Arrows indicate representative bands that are weaker in lig than the wild type at 28°C.
(B) Immunoblot analysis with the anti-PDI antibody. Proteins were extracted from the seedlings of the wild type (Ler) and lig mutant grown for 6 d at 18 or 28°C. A portion of the protein sample prepared from the wild type (Ler, 28°C) was incubated in the presence of Endo H or PNGase F for digestion of N-glycans before SDS-PAGE. Each lane contains proteins equivalent to the extract from 10 mg (fresh weight) of seedlings.
[See online article for color version of this figure.]