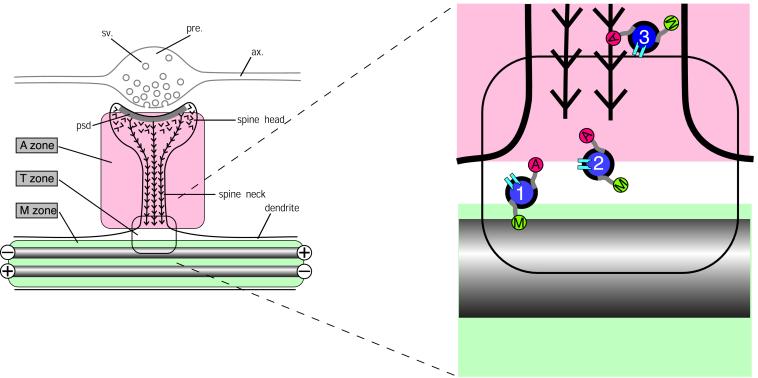
Figure 5.
Hypothetical scheme for the partitioning of cytoskeletal microdomains between shaft and spine in dendrites. (Left) Part of dendrite in the region of a spine synapse. The axonal component (ax.), with its swollen presynaptic (pre.) bouton containing synaptic vesicles (sv.) is outlined in gray. It forms a synapse at the tip of a dendritic spine head. Inside the spine head the junctional region is marked by the postsynaptic density (psd.), a complex of scaffolding proteins that acts as the platform for assembling functional molecules such as neurotransmitter receptors and ion channels. The cytoskeleton of the dendritic spine is composed of actin filaments (barbed lines) that are inserted into the psd. The cytoskeleton of the underlying dendrite consists predominantly of microtubules (gray rods), which in dendrites are bidirectionally oriented so that some have the plus ends distally and others the minus end distally as indicated. This distribution of cytoskeletal filaments demarcates three cytoplasmic zones, an M zone in the dendrite shaft, where microtubules predominate, an A zone in the dendritic spine, where actin filaments predominate, and a T, or transition, zone. (Right) The expanded diagram shows the relationship of these zones to the delivery of materials to the synaptic domain as suggested by current evidence. Transport vesicles (blue filled circles) carry cargoes of functional molecules, such as NMDA receptors (pale blue symbols), bound for the postsynaptic membrane. These vesicles bear both microtubule-dependent (M, kinesin and dynein) and actin-dependent (A, myosin) motor molecules. Transitory detachment of kinesin and dynein from microtubule tracks provides the opportunity for the myosin motors of transport vesicles to engage with the actin filaments of dendritic spines along which they travel to the synaptic domain. Single chevrons in the vicinity of the postsynaptic membrane represent the presence of labile actin filaments in this zone.