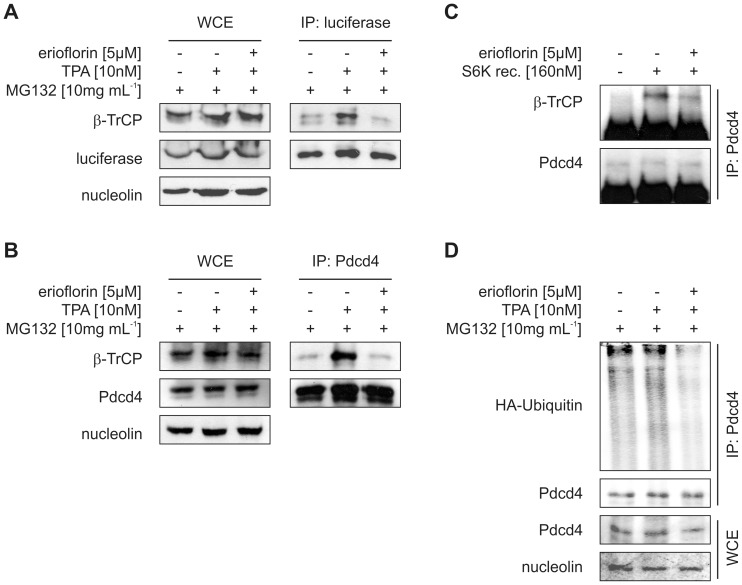
Figure 3. Erioflorin stabilizes Pdcd4 by interfering with β-TrCP1.
(A) Stably Pdcd4(39–91)luc expressing or (B) wildtype HEK293 cells were transfected with a β-TrCP1 expression vector one day prior to the experiment. Transfected cells were treated for 8 h with DMSO or TPA (10 nM) with or without erioflorin (5 µM) in the presence of the proteasome inhibitor MG132 (10 mg mL–1). Pull-down of luciferase (A) or Pdcd4 (B) out of whole-cell extracts was performed using specific antibodies. Subsequently, immunoprecipitated (IP) proteins and whole cell extracts (WCE) were subjected to western analysis and probed with the indicated antibodies. (C) In vitro-transcribed/translated β-TrCP1 and Pdcd4 proteins were co-incubated for 90 min at 30°C with or without recombinant p70S6K1 in the absence or presence of erioflorin (5 µM). Pdcd4 protein was immunoprecipitated and β-TrCP1 binding to Pdcd4 was analyzed by western analysis. (D) HEK293 cells were transfected with a plasmid expressing HA-tagged ubiquitin. Transfected cells were treated for 8 h with DMSO or TPA (10 nM) with or without erioflorin (5 µM) in the presence of the proteasome inhibitor MG132 (10 mg mL–1). Proteins co-immunoprecipitated with endogenous Pdcd4 and whole-cell extracts were analyzed by western analysis with the indicated antibodies. All blots are representative for at least three independent experiments.