Abstract
SUMMARY
Direct inhibition of thrombin has a beneficial effect on intestinal radiation toxicity. A small molecule inhibitor of protease-activated receptor 1 (PAR1), the most relevant receptor for thrombin, was administered to rats undergoing localized, fractionated irradiation of a segment of small intestine. Exogenous administration of the PAR1 inhibitor substantially ameliorated acute intestinal radiation mucositis, but did not interrupt the subsequent development of intestinal radiation fibrosis.
Purpose
Inhibition of thrombin ameliorates intestinal radiation injury. Protease-activated receptor 1 (PAR1), is the most relevant receptor for thrombin signaling and is not expressed on rat platelets. We used a specific small molecule inhibitor of PAR1 signaling to determine whether the beneficial effect of thrombin inhibition on radiation enteropathy development is due to inhibition of blood clotting or to cellular (PAR1-mediated) thrombin effects.
Methods and Materials
Rats underwent fractionated X-irradiation (5 Gy x 9) of a 4-cm small bowel segment. Early radiation toxicity was evaluated in rats receiving PAR1 inhibitor (SCH602539, 0, 10, or 15 μg/kg/d) from 1 day before to 2 weeks after the end of irradiation. The effect of PAR1 inhibition on development of chronic intestinal radiation fibrosis was evaluated in animals receiving SCH602539 (0, 15, or 30 μg/kg/d) until 2 weeks after irradiation, or continuously until termination of the experiment 26 weeks after irradiation.
Results
PAR1 blockade ameliorated early intestinal toxicity, with reduced overall intestinal radiation injury (p=0.002), number of myeloperoxidase-positive (p=0.03) and proliferating cell nuclear antigen-positive (p=0.04) cells, and collagen III accumulation (p=0.005). In contrast, there was no difference in delayed radiation enteropathy in either the 2- or 26-week administration groups.
Conclusion
Pharmacological blockade of PAR1 appears to reduce early radiation mucositis, but does not affect the level of delayed intestinal radiation fibrosis. Early radiation enteropathy is related to activation of cellular thrombin receptors, whereas, platelet activation or fibrin formation may play a greater role for the development of delayed toxicity. Because of the favorable side effect profile, PAR1 blockade should be further explored as a method to ameliorate acute intestinal radiation toxicity in patients undergoing radiation therapy for cancer and to protect first responders and rescue personnel in radiological/nuclear emergencies.
Keywords: Intestines, radiation injuries, thrombin, thrombin receptors, protease-activated receptors
INTRODUCTION
The intestine is an important dose-limiting organ during abdominal and pelvic radiotherapy. Despite advances in treatment delivery techniques, intestinal radiation injury (radiation enteropathy) remains an important obstacle to cancer cures and continues to adversely affect the quality of life of many cancer survivors. Clinically, intestinal radiation toxicity occurs as early (acute) or delayed (chronic) radiation enteropathy with related, but different underlying mechanisms. Pathologically, early radiation enteropathy (“radiation mucositis”) is characterized by epithelial barrier breakdown and mucosal inflammation, whereas, delayed radiation enteropathy exhibits prominent vascular sclerosis and intestinal wall fibrosis.
Radiation enteropathy is the result of complex interplay among many dose- and time-dependent pathophysiological processes, including inflammation, epithelial regeneration, tissue remodeling, and collagen deposition, as well as activation of the coagulation system and, notably, endothelial dysfunction. Endothelial cells are mechanistically involved in early and delayed radiation responses in many normal tissues, including the intestine (1–3). Exposure to ionizing radiation elicits profound changes in the endothelium, commonly referred to as “endothelial dysfunction”. Loss of thromboresistance due to downregulation of thrombomodulin and expression of tissue factor, resulting in increased formation of thrombin, is a hallmark feature of radiation-induced endothelial dysfunction (1, 3, 4).
Thrombin is a multifunctional serine protease that plays a central role in blood clotting by converting fibrinogen to fibrin and activating platelets. In addition, thrombin also regulates cell proliferation, inflammation, and tissue remodeling through activation of protease-activated receptors (PARs) (5), a family of four G-protein coupled receptors, PAR1, PAR2, PAR3, and PAR4. PAR1 is the most biologically relevant in inflammation and fibrosis and is prominently upregulated in irradiated intestine, suggesting a role in radiation enteropathy (3).
Preclinical and clinical studies performed in our and other laboratories demonstrate that so-called “endothelial-oriented” interventions can ameliorate radiation toxicity in several different normal tissues. We have also shown that there is increased thrombin formation, fibrin deposition, and upregulation of PAR1 in irradiated intestine (3, 4). While direct inhibition of thrombin ameliorates both early and delayed intestinal radiation injury (4), it is not known to what extent this benefit is due to suppression of thrombin-mediated blood clotting (conversion of fibrinogen to fibrin and stimulation of platelet aggregation) and/or to suppression of PAR1-mediated cellular effects. Because PAR1 is absent in rat platelets (6), this question was addressed using a specific small-molecule PAR1 inhibitor, SCH602539, in a preclinical rat model of radiation enteropathy. The results suggest that, while PAR1 is indeed involved in the development of early radiation toxicity (radiation mucositis), non-PAR1 mediated thrombin effects appear to be important in the mechanisms of development of chronic intestinal radiation fibrosis.
MATERIALS AND METHODS
Experimental Design
A total of 120 male Sprague-Dawley rats, 43–49 days of age (Harlan, Indianapolis, IN) were housed in conventional cages with free access to tap drinking water and standard mouse chow. All experimental protocols were approved by the Institutional Animal Care and Use Committee.
The rat surgical model for localized small bowel irradiation was prepared as described previously (7). Briefly, rats underwent bilateral orchiectomy, and a loop of distal ileum was sutured to the inside of the left part of the empty scrotum. The model creates a “scrotal hernia” that contains a 4-cm loop of small intestine that can be irradiated locally without additional surgery. The intestine remains technically within the abdominal cavity, and the surgical procedure does not cause appreciable long-term structural, functional, cellular or molecular alterations. The model minimizes manipulation during irradiation and produces radiation-induced changes similar to those seen clinically. This model has been extensively used and validated in our laboratory.
After 3 weeks of postoperative recovery, each rat was anesthetized with isoflurane inhalation, and the transposed bowel segment within the ‘”scrotal hernia” was exposed to once daily 5.0-Gy fractionated irradiation for 9 days using a Seifert Isovolt 320 X-ray machine (Seifert X-Ray Corporation, Fairview Village, PA), operated at 250 kVp and 15 mA, with 3 mm of added aluminum filtration. The resulting half-value layer was 0.85 mm Cu, and the dose rate was 4.49 Gy/min. The radiation regimen was based on data from previous experiments and was designed to elicit moderate to severe radiation enteropathy.
A potent, selective PAR1 antagonist, SCH602539, was provided by Schering-Plough Research Institute (Kenilworth, NJ). It is active both orally and subcutaneously; however, pharmacokinetics show that the compound lasts longer when injected, although it reaches peak plasma concentration more slowly (Supplementary Figure 1). To obtain the desired longer half-life in plasma, the compound was administered subcutaneously. The compound was dissolved in 0.4% of methylcellulose (Sigma-Aldrich, St. Louis, MO).
A schematic of the experimental design is shown in Figure 1. Each rat provided an irradiated and a (proximal) non-irradiated control segment of small intestine. The group sizes were based on expected effect size and attrition during the observation period.
Figure 1. Experimental design.
Experimental groups used to investigate the influence of SCH602539 on early and delayed radiation enteropathy.
The arrow indicates the period of irradiation, the dark shaded area represents the period of drug/vehicle administration, while the light shaded area represents observation without drug treatment.
To test whether administration of PAR1 antagonist attenuates early radiation-induced intestinal injury, 30 rats were randomly assigned to three groups (10 per group): vehicle, 10mg/kg/day of SCH602539, or 15mg/kg/day of SCH602539. The methylcellulose vehicle and SCH602539 were given subcutaneously 1 day before the start of radiation, during irradiation (9 days), and for 14 days thereafter. Animals were euthanized 2 weeks after the completion of irradiation.
To ascertain whether the short-term administration of PAR1 antagonist could interrupt the chronic fibroproliferative process in the irradiated intestine, 45 rats were randomly assigned to 3 groups (15 per group) for administration of vehicle, 15mg/kg/day of SCH602539, or 30mg/kg/day of SCH602539. The vehicle and SCH602539 were given subcutaneously 1 day before the start of radiation, during irradiation (9 days), and 14 days after the completion of irradiation. Animals were euthanized 180 days after the completion of irradiation.
Finally, to determine whether long-term administration of SCH602539 could reduce delayed radiation-induced injury, 45 rats were randomly assigned to 3 groups (15 per group), vehicle, 15mg/kg/day, or 30mg/kg/day of SCH602539. The vehicle and SCH602539 were given subcutaneously 1 day before the start of radiation, during irradiation (9 days), and 170 days after the completion of irradiation. Animals were euthanized 180 days after the completion of irradiation.
Assessment of the Intestinal Radiation Response
After euthanasia, specimens of irradiated and unirradiated intestine were procured and fixed in methanol-Carnoy’s solution for histological, morphometric, and immunohistochemical studies. The time points used in this study (2 weeks and 26 weeks) are representative of acute (early) and chronic (delayed) radiation enteropathy in our model system (7).
Quantitative histopathology and morphometry
Radiation injury score
The overall severity of structural radiation injury was assessed using the radiation injury score (RIS) system (see Supplementary Material). The RIS is a composite histopathological scoring system that provides a global measure of the severity of structural radiation injury. It has been extensively used and validated in our laboratory (8). All specimens were evaluated in a blinded fashion by two separate researchers.
Mucosal surface area
A decrease in the surface area of the intestinal mucosa is a sensitive parameter of small bowel radiation injury (8). Mucosal surface area was measured using a stereologic method as described by Baddeley (9).
Thickness of the intestinal wall and subserosa
Intestinal wall thickening is a measure of both reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia. Intestinal wall thickness and subserosal thickness were measured with computer-assisted image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). All measurements were done with a 10X objective lens. A total of 5 areas, 500 μm apart, were chosen for the measurement, with 3 measurements taken per area.
Quantitative immunohistochemistry and image analysis
Immunohistochemistry (see Supplementary Material) and computer-assisted image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) were used to assess the following established indicators of intestinal radiation injury: 1) neutrophil infiltration; 2) proliferation rate of intestinal smooth muscle cells; 3) deposition of collagen types I and III in the intestinal wall; and 4) expression of extracellular matrix-associated transforming growth factor-β (TGF-β) as described in detail and validated previously (10).
Myeloperoxidase
Neutrophils are an indicator of acute inflammation in the irradiated intestine. Myeloperoxidase enzymatic activity in leukocytes correlates directly with neutrophil number (r = 0.99), and myeloperoxidase activity in tissue extract correlates directly with neutrophil infiltration assessed histologically. The number of myeloperoxidase-positive cells in 20 fields (40X magnification) was determined.
Smooth muscle cell proliferation
In the intestine, collagen is mainly produced by intestinal smooth muscle cells, rather than by fibroblasts. Intestinal smooth muscle cell proliferation rate is very low at baseline, but increases steeply after irradiation. The numbers of total smooth muscle cells and PCNA-positive smooth muscle cells were determined in 20 fields at 40X magnification using color thresholding and normalizing PCNA-positive smooth muscle cells per thousand smooth muscle cells.
Collagen deposition
In the irradiated intestine, accumulation of collagen type I and, more markedly, accumulation of collagen type III occurs as reactive changes after only 2 weeks. Collagen type I or collagen type III immunoreactivity was determined in 20 fields (40X magnification), according to procedures established by Raviv et al. (11) and adapted to our model system.
Expression of TGF-β
TGF-β is overexpressed in many fibrotic conditions, including radiation fibrosis, and is mechanistically involved in radiation enteropathy (10). Areas relatively positive for TGF-β were determined in 20 fields (40X) according to procedures described by Raviv et al. (11) and adapted to our model system.
Statistical Methods
Sample size calculation was performed with PASS 2000 for Windows (NCSS, Kaysville, UT). Differences between experimental groups and variability for the early and delayed endpoints was derived from similar experiments conducted in our laboratory and used for calculations, making sure statistical power was at least 0.8. Statistical data analysis was performed with the software package NCSS2007 for Windows (NCSS, Kaysville, UT). Differences in endpoints between groups were assessed with multiple regression analysis (for dose-dependence). A p-value less than 0.05 were considered statistically significant.
RESULTS
Effect of PAR1 Antagonist on Early and Delayed Radiation Enteropathy in Rats
Radiation-induced histopathologic changes in the vehicle (control) group were similar to those observed in other studies performed in our laboratory (8). Early alterations (2 weeks) consisted mainly of mucosal injury (as measured by mucosal surface area), reactive intestinal wall thickening (as measured by intestinal wall thickening), and inflammatory cell infiltration (as measured by number of myeloperoxidase-positive cells). The delayed changes (26 weeks) included loss of mucosal surface area, increased number of myeloperoxidase-positive cells, smooth muscle cell proliferation, and excessive deposition of collagens and TGF-β in the intestinal wall (p<0.001 for all parameters, data not shown).
Administration of the PAR1 antagonist had no significant effect on normal intestinal structural or molecular parameters in the non-irradiated, proximal gut segment (p>0.05 for all parameters, data not shown).
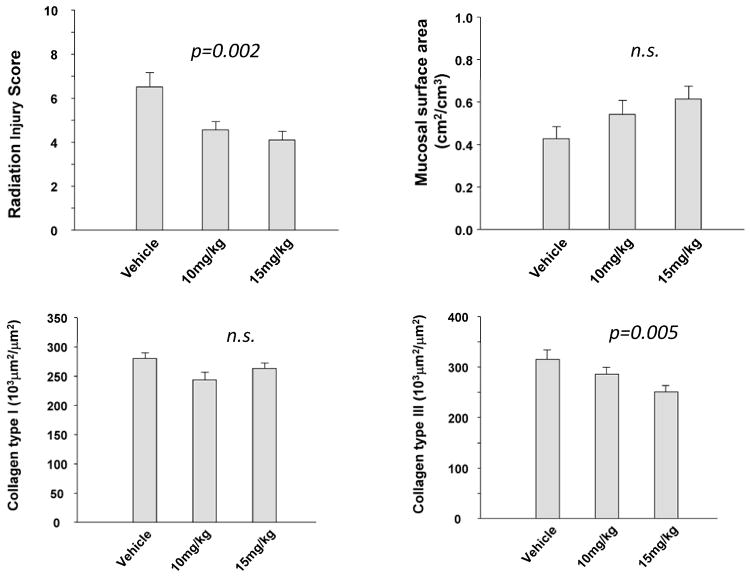
Two weeks after irradiation, SCH602539 administration was associated a reduction in Radiation Injury Score (p=0.002), number of myeloperoxidase-positive cells (p=0.03), intestinal smooth muscle proliferation (p=0.04), and collagen III immunoreactivity (p=0.005) (Figures 2–3 and Supplementary Material) in a dose-dependent manner. On the other hand, the differences in intestinal wall and serosal thickness, mucosal surface area, extracellular matrix-associated TGF-β expression, and collagen 1 deposition did not reach statistical significance. Representative histochemically and immunohistochemically stained images from the vehicle treated group and from rats treated with SCH602539 are shown in Figure 4–5 and in Supplementary Figure 2.
Figure 2. Effect of PAR1 blockade on early structural alterations and intestinal collagen accumulation.
Radiation injury score, mucosal surface area, and accumulation of collagen types I and III, 2 weeks after irradiation (N=10 per group).
Figure 3. Effect of PAR1 blockade on development of early radiation mucositis.
Myeloperoxidase, smooth muscle cell proliferation, and extracellular TGF-β immunoreactivity levels 2 weeks after the end of the radiation schedule (N=10 per group).
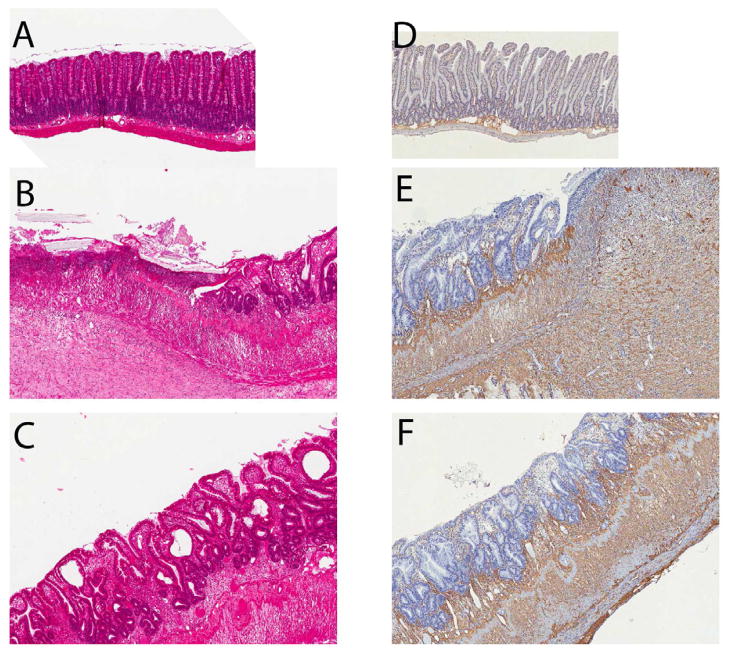
Figure 4. Representative histological images of control (unirradiated) intestine and early intestinal mucositis in rats treated with vehicle or PAR1 inhibitor.
Hematoxylin-eosin staining (A, B, and C): Unirradiated intestine (A) exhibits normal architecture. In contrast, 2 weeks after the end of a course of fractionated irradiation (9 daily fractions of 5 Gy) irradiated intestine shows extensive ulceration, substantial mucositis, and reactive fibrosis in the submucosa and smooth muscle cell layers (B). Treatment with SCH602539 15 mg/kg/d (C) ameliorates the histological evidence of radiation injury with preservation of the intestinal epithelium and less reactive fibrosis. Collagen III immunohistochemical staining (D, E, and F): Minimal collagen immunoreactivity in unirradiated intestine (D), localized mainly in the submucosa. Irradiated intestine (E) shows substantial accumulation of collagen III in the thickened submucosa and reactive fibrotic smooth muscle cell layers, which is somewhat ameliorated in intestine from SCH602539-treated rats (F).
Original magnification 3.5X for all images.
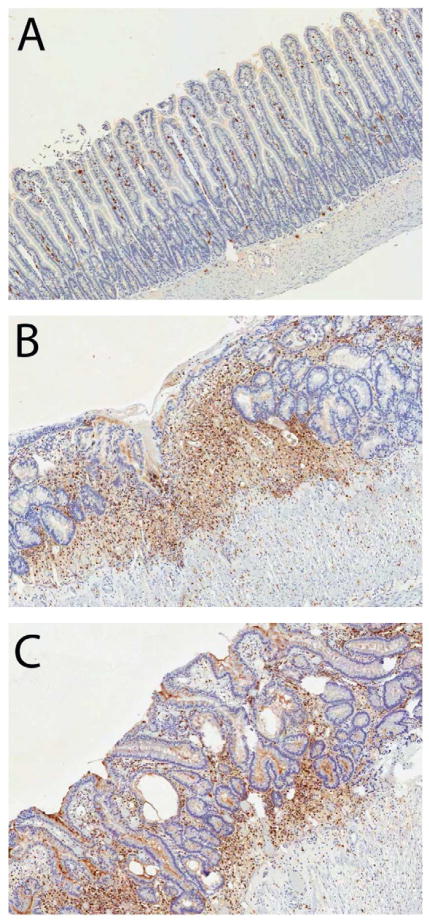
Figure 5. Influence of the PAR1 inhibitor on accumulation of mucosal myeloperoxidase-positive cells.
Unirradiated intestine showing scattered myeloperoxidase-positive cells in the mucosa (A). Irradiated intestine from vehicle-treated rat exhibiting substantial myeloperoxidase immunoreactivity (B). Irradiated intestine from a SCH602539-treated rat (C) showing better preservation of the epithelium and fewer myeloperoxidase-positive cells compared with the vehicle-treated control.
Original magnification 5X.
In contrast, neither short-term administration of PAR1 antagonist (administration until two weeks after radiation) nor long-term administration (daily administration until 26 weeks after radiation) was associated with a statistically significant difference in intestinal structural injury or difference in the aforementioned cellular or molecular parameters (Supplementary Figures 3–5).
DISCUSSION
Our data strongly suggest that cellular PAR1 signaling is involved in early radiation enteropathy development (i.e., radiation mucositis) but not in delayed injury (i.e., fibrosis). These findings have substantial translational significance because 1) PAR1 inhibition has substantially fewer side effects than direct thrombin inhibition (12), and 2) PAR1 blockade inhibits tumor growth and metastasis (reviewed in 13) and thus blocking PAR1 to reduce normal tissue toxicity would not be associated with concerns about tumor protection. The PAR1 inhibitor used in the present study, SCH602539, is an analog of vorapaxar (SCH530348) (14), a PAR1 antagonist that recently underwent phase 3 clinical development in heart disease. The main adverse effect of the PAR1 antagonist in humans (where PAR1 is present on platelets) is increased risk of bleeding.
Thrombin is a pluripotent serine protease that plays a critical role in blood clotting by converting fibrinogen to fibrin and by activating platelets after tissue injury. In addition to these procoagulant effects, thrombin involves the recruitment and trafficking of inflammatory cells and is a potent mitogen for a number of cell types, including endothelial cells, fibroblasts, and smooth muscle cells (5). Most cellular effects elicited by thrombin are mediated via PAR1. Thrombin activates PAR1 by cleaving the NH2-terminal sequence of the extracellular exodomain. The cleavage event results in a “new” amino terminal sequence, which serves as a so-called “tethered ligand” and binds intramolecularly to the ligand binding site to trigger trans-membrane signaling.
PAR1 is abundantly expressed throughout the gastrointestinal tract, and is strongly overexpressed in radiation-induced intestinal injury (3). PAR1-mediated responses include increased vascular permeability; release of vasoactive mediators; expression of adhesion molecules; and production of proinflammatory cytokines. PAR1 activation in the intestine induces epithelial apoptosis and loss of barrier function (15), and intracolonic administration of PAR1 agonists promotes local inflammatory responses (16). The results from the present study suggest that early intestinal radiation injury (“radiation mucositis”) is partly mediated by PAR1 activation, and that blocking PAR1 activation can ameliorate early intestinal toxicity.
The mechanisms by which PAR1 blockade reduces early radiation mucositis are not necessarily related to endothelial cells. PAR1 is not only expressed by endothelial cells, but also by the intestinal epithelium, immune cells, and a variety of stromal cells in the intestine. For example, PAR1 activated by mast cell proteases elicits a strong response in enteric neurons and thus may represent an important neural pathway in the radiation enteropathy development (reviewed in 17).
PAR1 is involved in a variety of fibroproliferative processes. Therefore, the finding that the PAR1 antagonist did not significantly affect delayed intestinal radiation fibrosis was somewhat surprising. PAR1 activation increases the expression of many profibrogenic mediators and PAR1 activation stimulates proliferation of fibroblasts and smooth muscle cells, as well as the production of pro-collagen. Moreover, (unpublished) experiments in PAR1-deficient animals showed a substantial reduction in delayed radiation toxicity. The obvious explanation is that there might be species differences (mice vs rats), differences due to the mode of irradiation (single dose vs fractionated irradiation), and/or that a degree of blocking of the receptor is needed that is difficult to achieve by pharmacological means.
A more likely explanation for the absence of an effect of SCH602539 and, in fact, a trend (albeit not statistically significant) toward worsening of delayed injury by PAR1 blockade is so-called “context dependent biased proteolysis” (18, 19). Hence, besides thrombin, activated protein C with involvement of the endothelial cell protein C receptor (EPCR) can also activate PAR1. Under these circumstances, PAR1 activation exhibits many protective (rather than detrimental) effects. For example, endogenous EPCR/aPC-PAR1 signaling exert anti-inflammatory properties in experimental colitis models (20). Ongoing experiments in our and other laboratories with variants of mouse activated protein C (wild type, anticoagulant, or PAR1 signaling) do indeed suggest that, depending on the context, activation of PAR1 by the protein C pathway may be biologically important.
CONCLUSIONS
SCH602539, a selective, small molecule, non-peptide antagonist of PAR1 appears to attenuate early intestinal radiation mucositis. This protective effect is likely due to inhibition of the cellular, receptor-mediated thrombin effects rather than to inhibition of blood clotting. The underpinnings of the lack of efficacy of SCH602539 in ameliorating delayed radiation fibrosis need further investigation. PAR1 blockade has antitumor effects and, compared to direct thrombin inhibition, far fewer side effects. Therefore, PAR1 blockade may be an attractive option for normal tissue protection in the clinic and for first responders, rescue workers, and cleanup personnel in radiological emergency situations.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (Grant CA-71382); Merck & Company, Inc.; and Veterans Healthcare Administration.
Footnotes
CONFLICTS OF INTEREST NOTIFICATION:
Madhu Chintala is an employee of Merck & Company, Inc., the company that produces SCH602539.
None of the other co-authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richter KK, Fink L, Hughes BM, et al. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy. Radiother Oncol. 1997;44:65–71. doi: 10.1016/s0167-8140(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 2.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and upregulation of protease-activated receptor 1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Zheng H, Ou X, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–2035. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 6.Ruef J, Kacharava A, Pohl J, et al. Indications for the presence of an atypical protease-activated receptor on rat platelets. Ann Hematol. 2000;79:604–611. doi: 10.1007/s002770000211. [DOI] [PubMed] [Google Scholar]
- 7.Hauer-Jensen M, Poulakos L, Osborne JW. Effects of accelerated fractionation on radiation injury of the small intestine: A new rat model. Int J Radiat Oncol Biol Phys. 1988;14:1205–1212. doi: 10.1016/0360-3016(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 8.Langberg CW, Sauer T, Reitan JB, et al. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 9.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Wang JR, Koteliansky VE, et al. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 11.Raviv G, Kiss R, Vanegas JP, et al. Objective measurement of the different collagen types in the corpus cavernosum of potent and impotent men: an immunohistochemical staining with computerized-image analysis. World J Urol. 1997;15:50–55. doi: 10.1007/BF01275157. [DOI] [PubMed] [Google Scholar]
- 12.Nadal-Wollbold F, Bocquet A, Bourbon T, et al. Protease-activated receptor 1 antagonists prevent platelet aggregation and adhesion without affecting thrombin time. Eur J Pharmacol. 2010;644:188–194. doi: 10.1016/j.ejphar.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Lopez MT, Gutierrez-Rodriguez M, Herranz R. Thrombin-activated receptors: promising targets for cancer therapy? Curr Med Chem. 2010;17:109–128. doi: 10.2174/092986710790112639. [DOI] [PubMed] [Google Scholar]
- 14.Chintala M, Strony J, Yang B, et al. SCH 602539, a protease-activated receptor-1 antagonist, inhibits thrombosis alone and in combination with cangrelor in a Folts model of arterial thrombosis in cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 2010;30:2143–2149. doi: 10.1161/ATVBAHA.110.203414. [DOI] [PubMed] [Google Scholar]
- 15.Chin AC, Vergnolle N, MacNaughton WK, et al. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci USA. 2003;100:11104–11109. doi: 10.1073/pnas.1831452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergnolle N, Cellars L, Mencarelli A, et al. A role for proteinase-activated receptor-1 in inflammatory bowel diseases. J Clin Invest. 2004;114:1444–1456. doi: 10.1172/JCI21689. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Wang J, Hauer-Jensen M. Neuroimmune interactions in the gut: potential target for mitigating or treating intestinal radiation injury. Br J Radiol. 2007;80:S41–S48. doi: 10.1259/bjr/33057885. [DOI] [PubMed] [Google Scholar]
- 18.Riewald M, Petrovan RJ, Donner A, et al. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 19.Ruf W. PAR1 signaling: more good than harm? Nat Med. 2003;9:258–260. doi: 10.1038/nm0303-258. [DOI] [PubMed] [Google Scholar]
- 20.Cenac N, Cellars L, Steinhoff M, et al. Proteinase-activated receptor-1 is an anti-inflammatory signal for colitis mediated by a type 2 immune response. Inflammatory Bowel Diseases. 2005;11:792–798. doi: 10.1097/01.mib.0000177506.71784.bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.