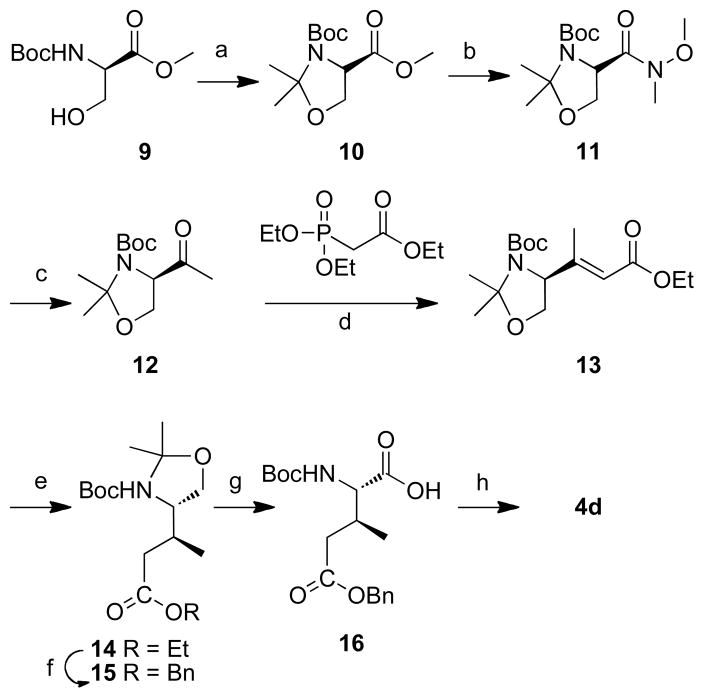
Scheme 2.
Reagents and conditions: a) 2,2-dimethoxypropane (10 eq.), BF3 Et2O (0.1eq.), acetone, rt, 2 h, 91%; b) 1) LiOH H2O (2.0 eq.), MeOH/H2O, rt, 4 h; 2) BOP (1.2 eq.), NH(OMe)Me HCl (1.25 eq.), Et3N (2.0 eq.), rt, 3 h, 70% for two steps; c) MeLi (1.7 eq.), THF, −78 °C, 1 h, 59%; d) triethyl phosphonoacetate (1.5 eq.), NaH (1.5 eq.), 0 °C, 24 h, 74%; e) 10% Pd•C, H2, MeOH/AcOEt, rt, 12 h, 97%; f) 1) LiOH H2O (3.0 eq.), THF/H2O, rt, 12 h.; 2) BnBr (1.5 eq.), NaHCO3 (2.0 eq.), DMF, rt, 24 h, 67% for two steps; g) 2.7 M Jones’ reagent (1.5 eq.), acetone, rt, 30 min, 89%; h) 1) 4M HCl in dioxane (10 eq.), 3 h; 2) Fmoc-OSu(1.5 eq.), NaHCO3 (2.0 eq.), THF – H2O (v/v 1:1), 12 h, 81% for two steps.