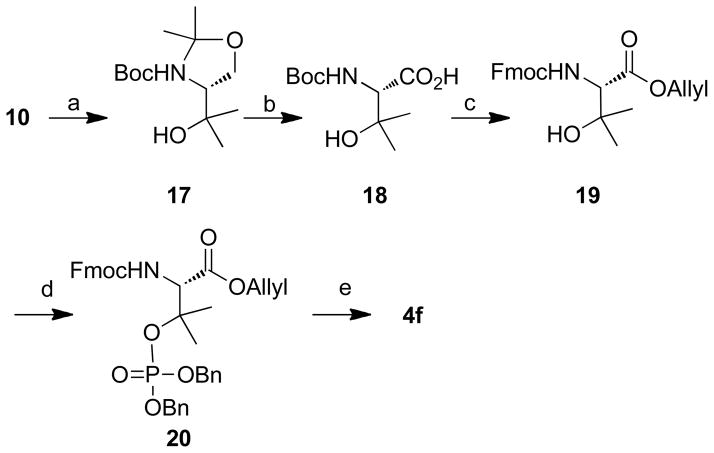
Scheme 3.
Reagents and conditions: a) Mg (6.0 eq.), MeI (7.5 eq.), Et2O, 0 °C, 30 min, 92%; b) Jones’ reagent (2.7 M, 1.5 eq.), acetone, 0 °C to rt, 1 h, 78%; c) 1) Allyl bromide (1.5 eq.), NaHCO3 (2.0 eq.), DMF, rt, 24 h, 60%; 2) 1M HCl in Et2O (10.0 eq.), rt, 3 h; 3) Fmoc-OSu (1.5 eq.), NaHCO3 (2.0 eq.), THF/H2O (1: 1 v/v), rt, 12 h, 85% for two steps; d) dibenzyl diisopropyl phosphoramidite (2.0 eq.), N-methylaniline trifluoroacetate (4.0 eq.), t-butyl hydroperoxide in n-octane (1.9 eq.), rt, 1 h, 90%; e) Pd(PPh3)4 (0.1 eq.), N-methylaniline (3.0 eq.), THF, rt, 1 h, 91%; 2) 10% Pd•C, 2, 2′-bipyridyl (0.5 eq.), H2, rt, 0.5 h, 60%.