Abstract
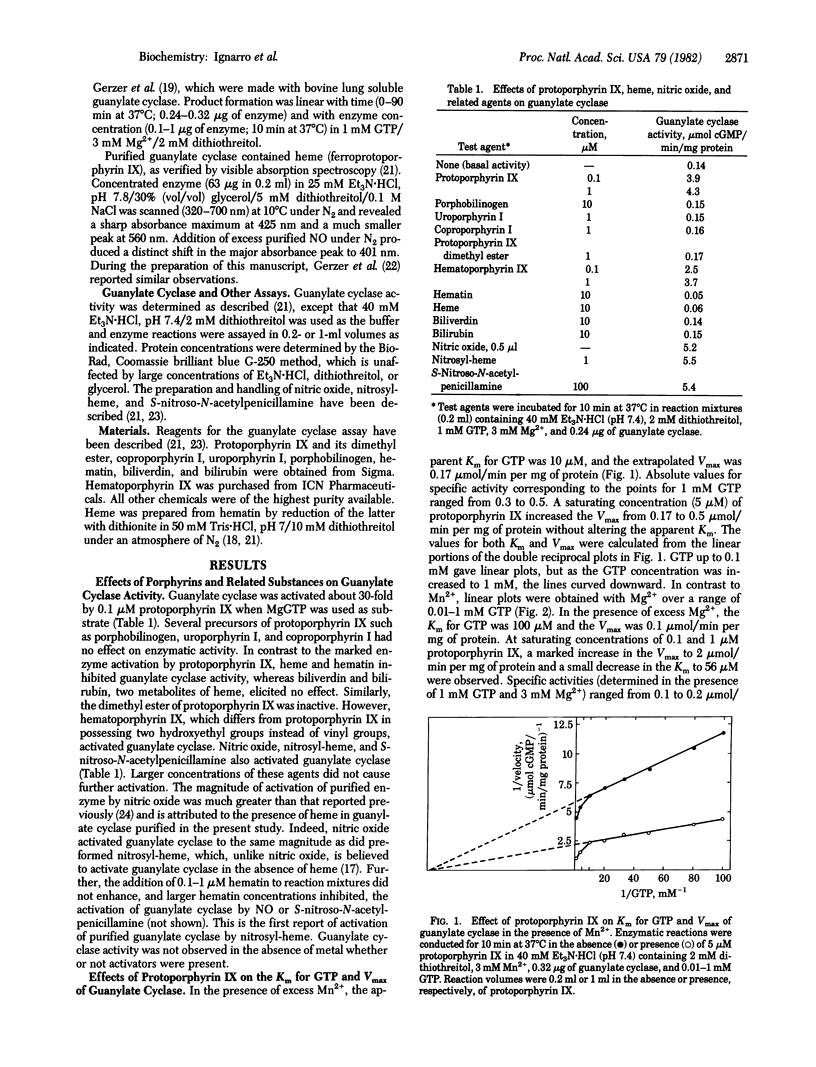
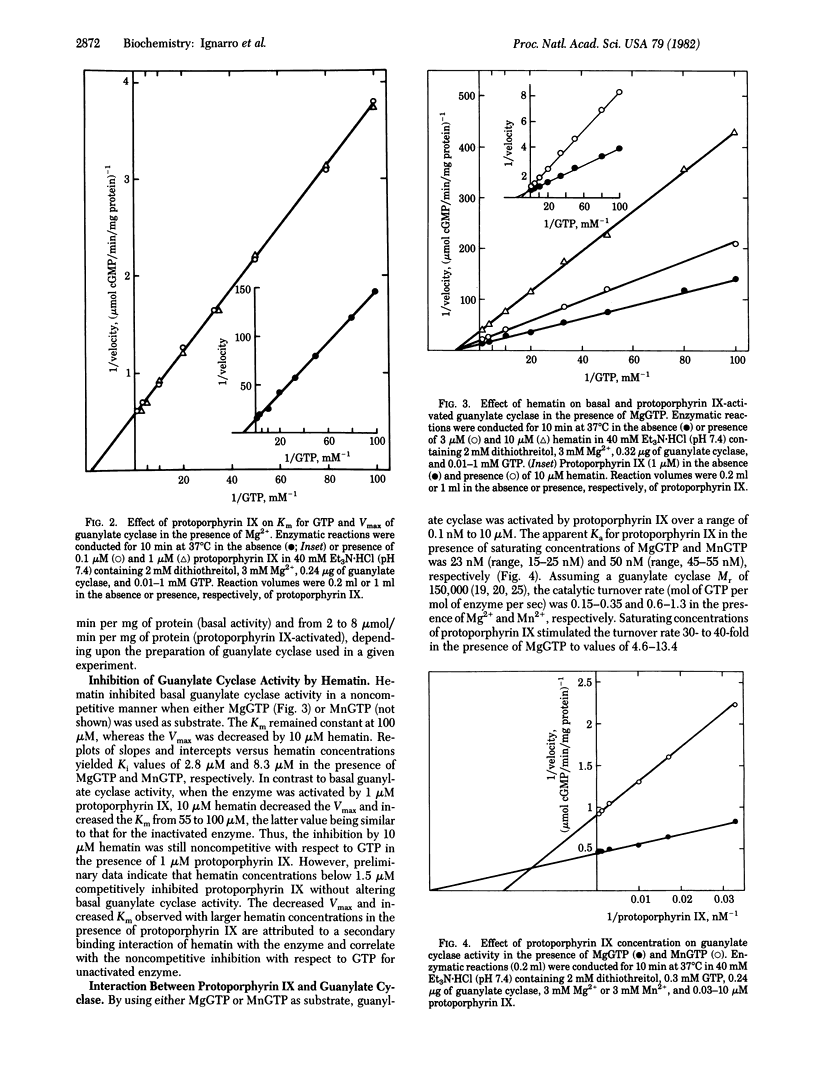
Soluble guanylate cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2] purified from bovine lung is markedly activated (30- to 40-fold) by protoporphyrin IX (Ka, 15-25 nM) and is inhibited by hematin (Ki, 3.7 microM) when MgGTP is used as substrate. Guanylate cyclase possesses specific activities (mumol of cGMP per min/mg of protein) of 0.1-0.2 (MgGTP) and 0.3-0.5 (MnGTP) and can attain values of 2-8 (MgGTP) or 1-1.4 (MnGTP) in the presence of protoporphyrin IX. Guanylate cyclase purified in this study contains heme and is activated by nitric oxide and nitrosyl-heme to the same magnitude as that by protoporphyrin IX. With the exception of hematoporphyrin IX, close structural analogs of protoporphyrin IX, including precursors and metabolites, do not activate guanylate cyclase. The insertion of iron into protoporphyrin IX to form heme or hematin renders the metalloporphyrin an inhibitor of unactivated or activated guanylate cyclase. The data suggest that protoporphyrin IX and heme could function to modulate guanylate cyclase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler J. M., Mittal C. K., Murad F. Purification of soluble guanylate cyclase from rat liver. Proc Natl Acad Sci U S A. 1979 Jan;76(1):219–222. doi: 10.1073/pnas.76.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme E., Graf H., Schultz G. Effects of sodium nitroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Adv Cyclic Nucleotide Res. 1978;9:131–143. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Activation of hepatic guanylate cyclase by N-methyl-N'-nitro-N-nitrosoguanidine. Effects of thiols, N-ethylmaleimide, and divalent cations. J Biol Chem. 1977 Aug 25;252(16):5804–5814. [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Calcium-independent modulation of cyclic GMP and activation of guanylate cyclase by nitrosamines. Science. 1976 Sep 3;193(4256):897–899. doi: 10.1126/science.7837. [DOI] [PubMed] [Google Scholar]
- Diamond J., Blisard K. S. Effects of stimulant and relaxant drugs on tension and cyclic nucleotide levels in canine femoral artery. Mol Pharmacol. 1976 Jul;12(4):668–692. [PubMed] [Google Scholar]
- Garbers D. L. Purification of soluble guanylate cyclase from rat lung. J Biol Chem. 1979 Jan 10;254(1):240–243. [PubMed] [Google Scholar]
- Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981 Sep 14;132(1):71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hofmann F., Schultz G. Purification of a soluble, sodium-nitroprusside-stimulated guanylate cyclase from bovine lung. Eur J Biochem. 1981 Jun 1;116(3):479–486. doi: 10.1111/j.1432-1033.1981.tb05361.x. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Ignarro L. J., Barry B. K., Gruetter D. Y., Ohlstein E. H., Gruetter C. A., Kadowitz P. J., Baricos W. H. Selective alterations in responsiveness of guanylate cyclase to activation by nitroso compounds during enzyme purification. Biochim Biophys Acta. 1981 Apr 3;673(4):394–407. doi: 10.1016/0304-4165(81)90471-2. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J., Baricos W. H. Evidence that regulation of hepatic guanylate cyclase activity involves interactions between catalytic site -SH groups and both substrate and activator. Arch Biochem Biophys. 1981 Apr 15;208(1):75–86. doi: 10.1016/0003-9861(81)90125-9. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- John M. E., Waterman M. R. Structural basis for the conformational states of nitrosyl hemoglobins M Saskatoon and M Milwaukee. Influence of distal histidine residues on proximal histidine-iron bonds. J Biol Chem. 1980 May 25;255(10):4501–4506. [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Activation of guanylate cyclase from rat liver and other tissues by sodium azide. J Biol Chem. 1975 Oct 25;250(20):8016–8022. [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Increases in cyclic GMP levels in brain and liver with sodium azide an activator of guanylate cyclase. Nature. 1975 Oct 23;257(5528):700–702. doi: 10.1038/257700a0. [DOI] [PubMed] [Google Scholar]
- Kon H., Kataoka N. Electron paramagnetic resonance of nitric oxide--protoheme complexes with some nitrogenous base. Model systems of nitric oxide hemoproteins. Biochemistry. 1969 Dec;8(12):4757–4762. doi: 10.1021/bi00840a016. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S., Wurm A., Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Morse R. H., Chan S. I. Electron paramagnetic resonance studies of nitrosyl ferrous heme complexes. Determination of an equilibrium between two conformations. J Biol Chem. 1980 Aug 25;255(16):7876–7882. [PubMed] [Google Scholar]
- Murad F., Lewicki J. A., Brandwein H. J., Mittal C. K., Waldman S. A. Guanylate cyclase: purification, properties, free radical activation, radiolabeling, and preparation of hybridoma antibodies. Adv Cyclic Nucleotide Res. 1981;14:229–239. [PubMed] [Google Scholar]
- Pfleiderer T. Na-nitroprussid. A very potent platelet desaggregating substance. Acta Univ Carol Med Monogr. 1972;53:247–250. [PubMed] [Google Scholar]
- Saxon A., Kattlove H. E. Platelet inhibition by sodium nitroprusside, a smooth muscle inhibitor. Blood. 1976 Jun;47(6):957–961. [PubMed] [Google Scholar]
- Schafer A. I., Alexander R. W., Handin R. I. Inhibition of platelet function by organic nitrate vasodilators. Blood. 1980 Apr;55(4):649–654. [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]


