Abstract
Kalirin-7 (Kal7), the major kalirin isoform in adult brain, plays a key role in the formation of dendritic spines in hippocampal/cortical neurons. Its role in the GABAergic medium spiny neurons (MSNs) of the nucleus accumbens (NAc) and striatum, the areas known to play a key role in the common reward pathway, is not as well understood. Although Kal7 expression in mouse NAc increased in response to cocaine, MSN dendritic spine density did not differ from that for the wild type in Kal7-null mice. Unlike wild-type mice, Kal7-null mice did not respond to cocaine with an increase in MSN dendritic spine density. To explore further the role of Kal7 in cocaine-induced alterations in MSN morphology, we turned to the rat. Based on immunostaining, both Kal7 and Kal12 are expressed at moderate levels in the MSNs of the NAc and striatum. Expression of Kal7 and Kal12 in MSNs of both areas increases after repeated cocaine treatments. Overexpression of Kal7 in cultured MSN neurons increases dendritic spine density, as observed in rats after long-term cocaine administration. Reducing endogenous expression of all major kalirin isoforms in cultured MSN neurons causes a decrease in total dendritic length and dendritic spine density. These data suggest that kalirin is essential for maintaining spine density in NAc MSNs under normal conditions and that Kal7 is an obligatory intermediate in the response of MSNs to repeated exposure to cocaine.
Introduction
Cocaine addiction is a chronic relapsing substance abuse problem associated with severe medical and psychosocial complications (Smith et al., 2009; Büttner, 2011). Despite decades of research, no effective treatment is available for cocaine addiction (Karila et al., 2008; Ross and Peselow, 2009; Schmidt and Pierce, 2010). It would be invaluable to have a better understanding of the molecular, cellular, and system-wide changes that accompany addiction. Repeated exposure to cocaine increases dendritic spine density in medium spiny neurons (MSNs) of the nucleus accumbens (NAc), key to the common reward pathway (Kolb et al., 2003; Shen et al., 2009; Kiraly et al., 2010b; Kim et al., 2011). Previous studies also demonstrated that repeated cocaine exposure enhances plasticity at incoming excitatory synapses onto MSNs (Schmidt and Pierce, 2010; Kalivas and Volkow, 2011). Surface glutamate receptor 1 expression and total levels of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor increase (Boudreau and Wolf, 2005). Dopamine receptor 2 surface expression (Conrad et al., 2010) and the level of transcription factors such as myocyte enhancer factor 2 and Δ-fosB (Nestler, 2008; Pulipparacharuvil et al., 2008) increase, as does cAMP response element-binding protein activation (Brown et al., 2011). Also well documented are changes in actin cycling, Homer1a, Kal7, NMDA receptors, and other postsynaptic proteins that regulate spine morphology and synaptic function (Szumlinski et al., 2008; Huang et al., 2009; Kourrich and Thomas, 2009; Moussawi et al., 2009; Shen et al., 2009; Kiraly et al., 2010b; Brown et al., 2011; Dobi et al., 2011; Kalivas and Volkow, 2011; Mains et al., 2011).
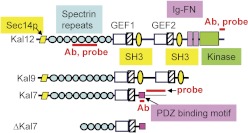
Through the use of different promoters and alternative splicing, the Kalrn gene generates multiple transcripts encoding functionally distinct proteins that include at least one Rho GDP/GTP exchange factor (GEF) domain (Johnson et al., 2000; Mains et al., 2011). The longest isoform of kalirin, Kal12, has a Sec14p domain, multiple spectrin-like repeats, two Rho GEF domains, two Src homology 3 motifs, Ig and fibronectin III motifs, and a kinase domain (Fig. 1). Kalirin plays a vital role in cytoskeletal organization, affecting process initiation and outgrowth plus dendritic spine formation (Rabiner et al., 2005; Ma, 2010). Kal7, the predominant isoform of kalirin in the adult brain (Fig. 1), is localized to the postsynaptic side of excitatory synapses and increases dendritic spine density when it is overexpressed in primary hippocampal and cortical neurons (Penzes et al., 2001; Ma et al., 2008b, 2011). In addition, Kal7 plays a key role in the formation of excitatory synapses onto hippocampal neurons (Ma et al., 2008a,b, 2010).
Fig. 1.
Kalirin isoforms. The major isoforms of kalirin in rat are diagrammed (Johnson et al., 2000). The regions targeted by the Kal7- and Kal12-specific probes and antisera used in these studies are indicated. Ab, antibody; SH3, Src homology 3.
The pathways through which cocaine alters synaptic plasticity in NAc MSNs are poorly understood. On the basis of the fact that CA1 hippocampal pyramidal neuron spine density is diminished in mice that cannot express Kal7, one might postulate a role for Kal7. However, NAc MSN spine density did not differ in wild-type and Kal7-null mice (Kiraly et al., 2010b). Although repeated cocaine treatment increased spine density in NAc MSNs in wild-type mice, no increase in spine density was observed in Kal7-null mice (Kiraly et al., 2010b). In this study, we assess expression and localization of specific isoforms of kalirin in the NAc and the dorsal striatum. The effects of overexpressing Kal7 and reducing endogenous kalirin expression in GABAergic MSNs indicate that multiple isoforms of kalirin play a role in the response of NAc MSNs to cocaine.
Materials and Methods
Animals and Tissue Preparation.
Adult male Sprague-Dawley rats (200–225 g) from Charles River Laboratories, Inc. (Wilmington, MA) were used. Male rats were injected with cocaine (20 mg/kg b.wt. i.p.) or saline between 9:00 and 10:00 AM for 8 consecutive days and were used 24 h after the last injection. All experiments were conducted in accordance with the guidelines established by the University of Connecticut Health Center Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996).
In Situ Hybridization.
Fresh brains from adult male rats were rapidly frozen on dry ice. Coronal sections (16 μm) were cut through the NAc in a cryostat and mounted on poly-l-lysine-coated slides. High specific activity antisense and sense riboprobes were made using [35S]UTP with T7 and T3 polymerase from the pBluescript-SKII(−) plasmid, respectively. The kalirin spectrin probes encoded part of the spectrin-like region common to all forms of kalirin. The Kal7 and Kal12 probes are specific to the 3′-untranslated region of Kal7 and the kinase domain of Kal12. In situ hybridization was performed as described previously (Ma et al., 2001, 2003).
Immunohistochemistry.
Animals were anesthetized with ketamine-xylazine and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate-buffered saline (Ma et al., 2001). Coronal sections (25 μm) through the NAc were cut. Kalirin antibody specificity was determined by replacement of an antibody with preimmune serum and by preincubation of the antibody with its antigen (10 μg/ml); staining was eliminated in both controls (Ma et al., 2001, 2008b). Sections were incubated overnight at 4°C with one of three primary rabbit polyclonal antibodies: kalirin antiserum JH 2582 (spectrin domain); affinity-purified Kal7 antiserum JH2958 (C terminus of Kal7); or affinity-purified Kal12 antiserum JH3226 (C terminus of Kal12). Primary antibodies were visualized by staining with a peroxidase-tagged second antibody followed by diaminobenzidine or with Cy3-conjugated donkey anti-rabbit IgG (The Jackson Laboratory, Bar Harbor, ME) as described previously (Ma et al., 2003, 2011).
Western Blot Analysis.
The NAc and striatum were dissected, and tissues were homogenized in 10 volumes of SDS lysis buffer containing phenylmethylsulfonyl fluoride and a protease inhibitor mixture (lima bean trypsin inhibitor, leupeptin, benzamidine, and pepstatin) with five strokes of a glass/Teflon homogenizer (Ma et al., 2008b) followed by boiling. Large debris was removed by centrifugation at 5000g for 5 min. Proteins fractionated by SDS gel electrophoresis were transferred to Immobilon-P membranes (Millipore Corporation, Billerica, MA) and visualized with one of several primary antisera [kalirin antisera as above; cdk5 (Millipore Corporation), 1:1000; p35 (Cell Signaling Technology, Danvers, MA), 1:1000; and Phospho-Thr34-DARPP-32 (Cell Signaling Technology), 1:500)] and the Pierce ECL Kit (Ma et al., 2003).
Preparation of Rat Nucleus Accumbens Organotypic Cultures.
NAc slices were prepared from P8 Sprague-Dawley rats as described previously (Ma et al., 2003). In brief, the NAc was dissected into ice-cold, sterile Gey's balanced salt solution (Sigma-Aldrich; St. Louis, MO) containing 0.5% glucose and then was sliced transversely at a thickness of 400 μm on a slice chopper. Slices were kept in ice-cold Gey's balanced salt solution and then placed onto 30-mm Millicell CM membrane inserts (Millipore Corporation) in Petri dishes with 1.1 ml of culture media containing 0.5× basal Eagle's medium, 0.25× Hanks' balanced salt solution, 0.25× horse heat-inactivated serum, 100 units/ml each penicillin and streptomycin, and 1 mM l-glutamine (Invitrogen, Carlsbad, CA). Slices were kept under 5% CO2 at 37°C, with half-medium changes at 1 day in vitro and every 3 days thereafter.
Preparation of Expression Vectors, DNA-Coated Gold Microcarrier Particles, and Biolistic Transfection.
The pEAK vectors encoding GFP and Myc-tagged Kal7 (Ma et al., 2003) were mixed (GFP/Kal7 = 1:2) before being coated onto gold particles. pCMS-GFP-Kal antisense and control pCMS-GFP-Trio antisense were described previously (Ma et al., 2003). Plasmid DNA was precipitated onto 1.0-μm gold microcarrier particles at a concentration of 1 μg of plasmid/mg gold, according to the Bio-Rad Laboratories (Hercules, CA) instruction manual. Plasmid-coated gold particles were resuspended in 0.05 mg/ml polyvinylpyrollidone in ethanol and dried onto gold-coated tubing (25 mg of gold particles/30 inches of tubing). Slices were transfected using the Helios Gene Gun System (Bio-Rad Laboratories) after 2 days in vitro. After transfection (72–96 h), slices were fixed with 4% paraformaldehyde and then processed for immunostaining using monoclonal myc antibody 9E10 and a polyclonal antibody to GFP (Nacalai Tesque, Kyoto, Japan), which was used to intensify the GFP signal, providing better evaluation of fine dendritic processes (Ma et al., 2003).
Image Analysis and Quantification.
Isolated GFP-positive neurons in organotypic slices were imaged using a LSM510 confocal microscope (Carl Zeiss Inc., Thornwood, NY). For quantification of total dendritic length, a stack of images (Z step, 1 μm) was taken using a 20× objective (0.7 digital zoom factor), and the entire neuron was visualized in three dimensions with Zmaris 3.2 (Bitolane AG, Zurich, Switzerland). Dendrites were traced using Neurolucida, and total dendritic length was calculated using Neurolucida (Ma et al., 2003). For spine quantification, stacks of confocal images (Z step, 0.2 μm) were taken using a 63× objective. Three-dimensional images were assembled with Zmaris, and spines were traced and quantified using Neurolucida (Ma et al., 2003). Data are presented as average ± S.E.M. Statistical analyses were performed with SPSS 10 (SPSS Inc., Chicago, IL). P values in the text are from Student's t test.
Results
Kalirin Is Expressed in the Neurons of the NAc and Dorsal Striatum.
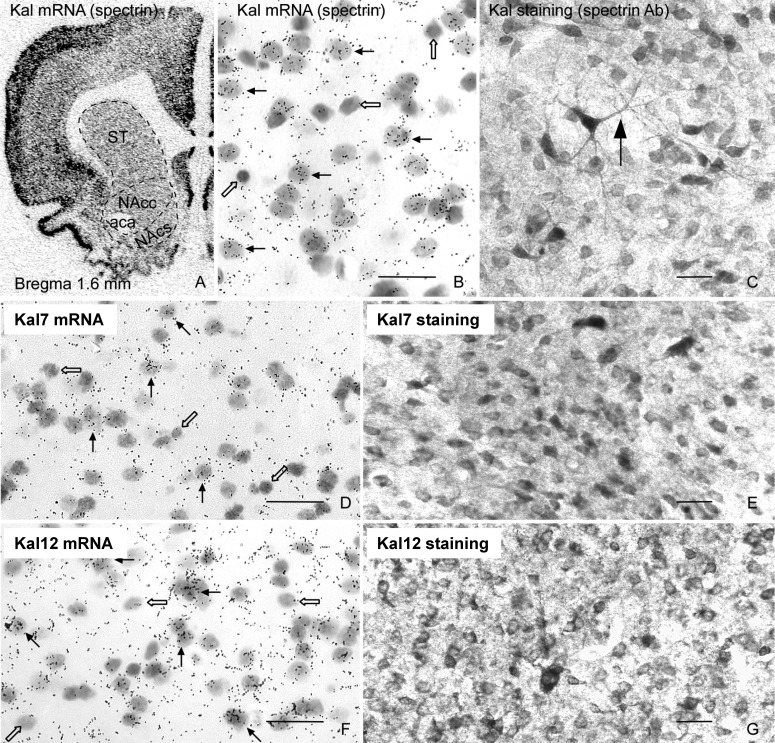
In situ hybridization with a Kal-spectrin probe (Fig. 1), which detects all major kalirin isoforms, shows widespread expression of kalirin (Fig. 2A). In situ hybridization with emulsion-dipped slides and immunocytochemical analysis both demonstrate that kalirin is expressed at similar levels throughout the NAc and dorsal striatum. Double immunostaining with antibodies specific to the kalirin spectrin domain, which detects all major kalirin isoforms (Fig. 1), and glial fibrillary acidic protein, a glial marker, shows that kalirin staining is absent from glial cells (not shown); this observation is in agreement with in situ hybridization data showing that not all cells in the striatum express kalirin (Fig. 2B, open arrows). Kalirin is also expressed in large neurons scattered throughout the striatum (Fig. 2C, arrow); the identity of these large neurons as cholinergic interneurons was confirmed by double immunostaining with antibodies specific to kalirin and choline acetyltransferase (not shown). In situ hybridization and immunostaining with probes and antibodies specific to the COOH terminus unique to Kal7 and ΔKal7 or the COOH terminus unique to Kal12 (Fig. 1) showed that most of the cells in the NAc and dorsal striatum express both Kal7 (Fig. 2, D and E) and Kal12 (Fig. 2, F and G). The MSNs of the NAc receive their dopaminergic input from the VTA, so the presence of kalirin in these dopaminergic projection neurons was also investigated (not shown). The results show that Kal7 is expressed at similar levels in most of the tyrosine hydroxylase-positive neurons of the VTA and substantia nigra (not shown).
Fig. 2.
Expression of kalirin in rat nucleus accumbens. In situ hybridization with 35S-labeled riboprobes specific to the spectrin-like region of kalirin (A and B), Kal7 (D), and Kal12 (F) shows that kalirin mRNAs are expressed in neurons in the core (NAcc) and shell (NAcs) regions of the NAc and in the dorsal striatum (ST). A, x-ray film (bregma 1.6 mm) (Ma et al., 2001). B, D, and F, emulsion-dipped slides. Immunostaining with antibodies specific to Kal-spectrin (C), Kal7 (E), and Kal12 (G) confirmed these findings. Immunostaining with Kal-spectrin antibodies was detected in the soma and long dendrites (C). B–G are from the core of the nucleus accumbens. Open arrows, negative cells; solid arrows, positive neurons. Scale bars, 20 μm. Sections were counterstained with cresyl violet acetate.
Kal7 and Kal12 Expression Increases in Response to Long-Term Cocaine in Most MSNs of the NAc and Dorsal Striatum.
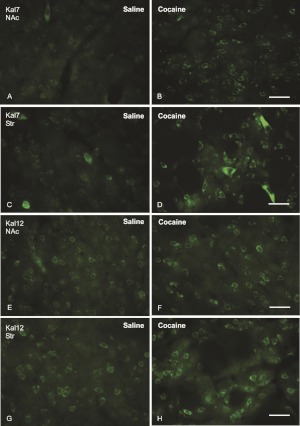
Repeated exposure to cocaine was shown to increase kalirin expression in the mouse NAc and dorsal striatum (Mains et al., 2011). Adult male rats received eight daily injections of saline or cocaine (20 mg/kg) and were sacrificed 24 h later. Treatment efficacy was verified by demonstrating increased expression of cdk5 and p35 mRNA and protein along with decreased levels of Phospho-Thr34-DARPP-32 (Supplement Fig. S1) (Bibb et al., 2001). The question of whether Kal7 expression in most MSNs or in only a subset responds to repeated cocaine was investigated by immunocytochemistry using antisera specific to Kal7 (Fig. 3, A–D) and Kal12 (Fig. 3, E–H). The data demonstrate that a large percentage of the Kal7-containing neurons (Fig. 3, A–D) and Kal12-containing neurons (Fig. 3, E–H) in the NAc and dorsal striatum show an increase in staining intensity in response to repeated administration of cocaine. The large cholinergic interneurons scattered throughout the dorsal striatum express high levels of Kal7 under control conditions but still show increased staining in response to long-term cocaine treatment (Fig. 3, C–D). Cocaine-induced increases in fluorescence intensity for Kal7 and Kal12 staining are in agreement with reverse-transcription-polymerase chain reaction data demonstrating an increase in mRNA levels (Supplement Fig. S2) and Western blot data demonstrating an increase in protein levels (Supplement Fig. S3).
Fig. 3.
Cocaine treatment increases Kal7 and Kal12 expression in the MSNs of the striatum (Str) and the NAc. Immunostaining with antibodies specific to the COOH termini of Kal7 and Kal12 showed that Kal7 (A–D) and Kal12 (E–H) staining increased in the neurons of the NAc core (A and B and E and F) and striatum (C and D and G and H) after 8 days of cocaine treatment (B, D, F, and H) in comparison with saline-injected controls (A, C, E, and G). NAc, images were taken from the NAc core. Scale bars, 40 μm.
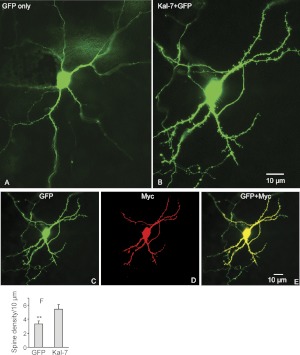
Kal7 Overexpression Increases the Density of Dendritic Spines in MSNs.
Expression of exogenous Kal7 has been shown to increase the density of dendritic spines in cultured hippocampal and cortical neurons (Penzes et al., 2001; Ma et al., 2008b). Organotypic slice cultures were used to determine whether the MSNs of the NAc would respond to exogenous Kal7 in the same manner. Slices prepared at postnatal day 8 were biolistically cotransfected with vectors encoding Myc-Kal7 and GFP at postnatal day 10 and examined 2 days later, at a time when the spine density and endogenous Kal7 levels in the animal are still low; control slices received vector encoding GFP. The results showed that expression of Myc-Kal7 caused a dramatic increase in spine density; the increase in spine density was apparent over the full length of the dendrite in NAc MSNs (Fig. 4, A–E) and the spine density is comparable to values found in vivo (Wang et al., 2012). Quantification revealed an approximately 50% increase in spine density in MSNs expressing Myc-Kal7 (Fig. 4F).
Fig. 4.
Overexpression of Kal7 in the MSNs of NAc increases spine density. NAc slices were prepared from postnatal day 8 rats and were biolistically transfected with a vector encoding GFP alone (A) or cotransfected with a vector encoding GFP plus a vector encoding Myc-tagged Kal7 (B) using a gene gun (GFP/Kal7 = 1:2 ratio). Neurons were fixed 48 h after transfection, doubly stained with polyclonal GFP (C) and monoclonal myc (D) antibodies, and visualized with Cy3-conjugated donkey anti-mouse IgG and fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG. Spine density was analyzed using Neurolucida as described previously (Ma et al., 2003) (F). **, quantification of spine density, p < 0.01, Student t test, n = 9. Scale bars, 10 μm.
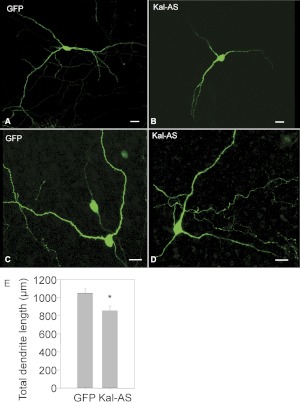
Reduced Expression of Kalirin Decreases Dendritic Length and Spine Density in NAc MSNs.
Kalirin expression was reduced using an antisense approach as described previously (Ma et al., 2003); the antisense vector used encodes approximately 1 kilobase of the Kal-spectrin domain (Fig. 1) and reduces levels of mRNA encoding all major full-length and Δ-isoforms of kalirin. Use of a dual promoter vector ensures expression of GFP in neurons expressing the antisense construct; controls were transfected with vector encoding GFP alone or GFP and a control antisense sequence. The MSNs in which kalirin expression was reduced still form dendrites (Fig. 5, A–D) but exhibit an ∼20% decrease in total dendritic length (Fig. 5E). The dendritic lengths in the P10 cultures are approximately 75% of those seen in adults in vivo (Wang et al., 2012). When examined at higher magnification, the effect of reducing kalirin expression on spine formation is apparent (Fig. 6, A–D); quantification reveals an almost 3-fold decrease in spine density (Fig. 6E). In previous work with hippocampal cultures, control vectors using the corresponding region of the kalirin homolog trio showed no changes in dendritic structure (not shown); antisense to the secretory granule enzyme peptidylglycine α-amidating monooxygenase produced a dramatic drop in peptidylglycine α-amidating monooxygenase levels with no change in dendritic structure (Ma et al., 2003).
Fig. 5.
Reduction of endogenous kalirin expression decreases total dendritic arbor length of MSNs in the NAc. NAc slices prepared from postnatal day 8 rats were biolistically transfected using pCMS vector encoding GFP alone (control, A and C) or pCMS vector encoding GFP and kalirin antisense (B and D) at 48 h in vitro. The efficiency of pCMS vector encoding GFP and kalirin antisense was determined previously (Ma et al., 2003). Subsequent immunostaining with GFP antibody was performed 48 h after transfection to intensify the GFP signal. Neurolucida was used to trace and calculate the total length of dendrites (E) as described previously. *, p < 0.05, Student's t test, n = 10. Scale bar (A–D), 15 μm.
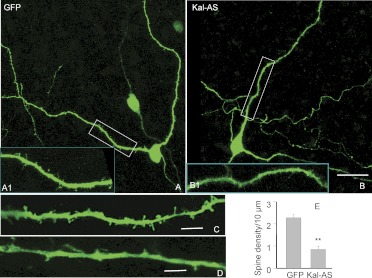
Fig. 6.
Reduction of endogenous kalirin expression decreases spine density on MSNs in the NAc. NAc slices (P8) were biolistically transfected with pCMS vector encoding GFP alone (A, A1, and C) or pCMS vector encoding GFP and Kal antisense (AS) (B, B1, and D) using a gene gun as described in the legend to Fig. 5. Slices were fixed with 4% paraformaldehyde 72 h after transfection and imaged with confocal microscopy. Insets A1 and B1 show higher magnification images of boxed areas in A and B, respectively. Scale bars (A and B), 15 μm; (C and D), 5 μm. Spine density was measured using Neurolucida as described previously (Ma et al., 2003) (E). **, p < 0.01, Student's t test, n = 10.
Discussion
Addiction is a complex response observed after repeated exposure to drugs of abuse. The effects of long-term exposure to cocaine are complex, widespread, and long-lasting. Because no effective treatment is available, it is essential to develop a better understanding of the molecular, cellular, and system-wide changes associated with addiction (Ross and Peselow, 2009; Kalivas and Volkow, 2011). The long-lasting nature of addiction leads to relapse and makes it especially difficult to treat. Structural changes at many of the synapses involved in addiction are thought to underlie these long-lived changes. Because dendritic spine morphology is largely controlled by the actin cytoskeleton, attention has turned to the pathways through which cocaine could alter the cytoskeleton. Small GTP-binding proteins of the Rho family play an important role in this process; they are activated by Rho GEFs and inactivated by Rho GTPase-activating proteins (Rossman et al., 2005). There are ∼60 Rho GEFs in the human genome, and about a dozen are found in significant amounts at the synapse (Kiraly et al., 2010a). We have focused on kalirin because Kal7KO mice, which lack the major adult brain isoform of kalirin, have an enhanced locomotor response to cocaine; although they show a normal place preference for food, Kal7KO mice show a decreased place preference for cocaine (Kiraly et al., 2010b). Spine density on the dendrites of Kal7KO MSNs is indistinguishable from that of the wild type. Whereas long-term cocaine produces an increase in spine density and spine area in wild-type mice, spine density fails to increase in Kal7KO mice, and spine area in the Kal7KO mice decreases in response to cocaine (Kiraly et al., 2010b).
Long-term exposure to cocaine is known to increase the expression of kalirin in mouse NAc (Kiraly et al., 2010b). A similar response was observed in rats, with increased expression of Kal7, Kal9, and Kal12 mRNA and protein in NAc and striatum (Supplemental Figs. S1–S3). In situ hybridization with isoform-specific probes revealed similar levels of expression of Kal7 and Kal12 mRNA in all medium spiny neurons (Fig. 2). Immunofluorescence studies with isoform-specific antisera confirmed this conclusion. In the NAc and striatum, expression of both Kal7 and Kal12 increased in the majority of the MSNs in response to long-term cocaine treatment (Fig. 4). The D1 and D2 receptor medium spiny neurons (D1- and D2-MSN) are known to play different roles in the response to cocaine. Knockout of D1 receptors abolishes the short-term locomotor response (Miner et al., 1995; Zhang and Xu, 2001; Karlsson et al., 2008), whereas D2 receptor knockout merely blunts the locomotor response (Welter et al., 2007). Ablation of the protein phosphatase regulatory subunit DARPP-32 in D1-MSNs blocks the locomotor response to short-term cocaine, whereas knocking out DARPP-32 in D2-MSNs increases the short-term locomotor response to cocaine (Bateup et al., 2010). Cocaine induces the appearance of new spines in both D1-MSNs and D2-MSNs (Kim et al., 2009), but the new spines remain elevated during withdrawal from cocaine only in D1-MSNs (Lee et al., 2006). D1, D3, and κ-opioid receptor levels increase after cocaine self-administration, whereas D2 receptor levels decrease (Mash and Staley, 1999; Conrad et al., 2010). Self-administration of cocaine is blocked by D1 antagonists and by genetic ablation of the D1 receptor (Orio et al., 2009).
Dopamine neurons in the VTA play a key role in cocaine addiction, projecting mainly to the NAc and prefrontal cortex (Lüscher and Malenka, 2011). The induction of cocaine sensitization and cocaine self-administration involves the enhancement of excitatory synapses in the VTA (Ungless et al., 2001; Borgland et al., 2004). The endogenous expression of Kal7 in the dopaminergic neurons of the VTA (not shown) puts Kal7 in the right place to play a role in cocaine-induced synaptic plasticity in these VTA neurons (Ungless et al., 2001; Sarti et al., 2007).
We used slice cultures to assess the ability of exogenous Kal7 to increase the formation of dendritic spines in MSNs. As seen in hippocampal and cortical neurons, spine density in MSNs increased in response to an increase in Kal7 expression; spines were uniformly distributed along the dendrites (Fig. 5). The cocaine-mediated increase in Kal7 expression could thus cause the increase in spine density observed in wild-type mice. Of interest, the new spines/synapses that appear after cocaine treatments are enriched in synaptic NR2B-containing NMDA receptors, whereas far fewer new spines/synapses are seen if NMDA receptors, specifically NR2B-containing NMDA receptors are blocked (Huang et al., 2009; Ren et al., 2010; Brown et al., 2011). The PH domain of Kal7 binds directly to the juxtamembrane region of NR2B (Kiraly et al., 2011), which in turn interacts with the actin cytoskeleton. Thus, the increase in Kal7 protein seen after cocaine treatment of rats and mice, combined with the direct interaction of Kal7 with NR2B, may be the immediate cause of the increased NR2B in the new spines. The importance of glutamate signaling in the response to cocaine has been emphasized in one recent review (Kalivas and Volkow, 2011). NMDA receptors are functional on both D1-MSN and D2-MSN (Valjent et al., 2005; Shi et al., 2011).
Kalirin expression in MSNs was reduced using an antisense construct that targets all full-length isoforms; in addition to a decrease in expression of Kal7, levels of Kal9 and Kal12, which also interact with NR2B, are reduced. In the MSNs with reduced levels of all of these isoforms of kalirin, the length of the dendritic arbor was reduced and spine density was diminished (Figs. 5 and 6). This would be expected from previous work on sympathetic neurons, because Kal7, Kal9, and Kal12 all increase both fiber numbers and branching complexity (May et al., 2002). However, overexpression of Kal9 in cortical neurons is reported to increase dendritic spine numbers, coupled with a small decrease in total dendrite length (Deo et al., 2012). A decrease in NR2B levels is observed in the hippocampus of Kal7KO mice. There is less NR2B subunit in cortical postsynaptic densities of Kal7KO mice than in control mice (Ma et al., 2008a). Many forms of long-term potentiation and long-term depression are NMDA receptor-dependent; hippocampal long-term potentiation and long-term depression were dramatically diminished in the Kal7KO mouse (Ma et al., 2008a; Lemtiri-Chlieh et al., 2011). The lack of Kal7 also leads to a lack of new dendritic spines in the NAc in response to cocaine (Kiraly et al., 2010b). Perhaps the new spines with additional NR2B receptor subunits normally observed in the NAc are protective in long-term cocaine use, and the lack of new NR2B-containing spines is the cause for the enhanced locomotor sensitization to cocaine administration.
The complex circuitry through which glutamatergic signaling plays a role in addiction means that changes in kalirin expression in dopaminergic neurons in the VTA, GABAergic MSNs in the NAc and striatum, and glutamatergic pyramidal neurons in the prefrontal cortex could play a role. The ability of kalirin, a cocaine-responsive gene, to interact with the NR2B subunit of the NMDA receptor may be one mechanism through which cocaine exerts long-term effects on cytoskeletal organization. NMDA receptors are linked to the actin cytoskeleton by both spectrin and α-actinin (Wyszynski et al., 1997; Wechsler and Teichberg, 1998). Tiam-1, another Rac GEF, binds to the NR1 subunit of NMDA receptors (Tolias et al., 2005), whereas RasGRF1, a GEF for both Ras and Rac, binds to the NR2B subunit. Like Kal7KO mice, RasGRFKO mice show decreased place preference for cocaine (Fasano et al., 2009). Synapses rich in both Kal7 and NR2B may be especially sensitive to long-term cocaine. The effects of cocaine are known to differ in men and women (Parylak et al., 2008). In rats, dendritic spine density and sensitivity to cocaine vary throughout the estrus cycle, with a greater response to cocaine when estradiol levels are high and spines are more numerous (Becker, 1999; Parylak et al., 2008). Kal7 levels in the hippocampus vary in a similar manner throughout the menstrual cycle, and elevated estradiol levels increase both Kal7 expression and dendritic spine density in the rat hippocampus and in cultured rat hippocampal neurons (Ma et al., 2011).
Supplementary Material
Acknowledgments
We thank Darlene D'Amato for tireless work on all aspects of this project.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA15464].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- MSN
- medium spiny neuron
- NAc
- nucleus accumbens
- Kal
- kalirin
- NDMA
- N-methyl-d-aspartate
- GEF
- guanine nucleotide exchange factor
- GFP
- green fluorescent protein
- VTA
- ventral tegmental area
- KO
- knockout.
Authorship Contributions
Participated in research design: Ma, Mains, and Eipper.
Conducted experiments: Ma, Huang, Xin, Yan, Mains, and Eipper.
Wrote or contributed to the writing of the manuscript: Ma, Mains, and Eipper.
References
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA 107:14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812 [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, et al. (2001) Effects of chronic exposure to cocaine are regulated by the neuronal protein cdk5. Nature 410:376–380 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. (2004) Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24:7482–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. (2005) Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25:9144–9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. (2011) A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 31:8163–8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner A. (2011) Review: The neuropathology of drug abuse. Neuropathol Appl Neurobiol 37:118–134 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME. (2010) Dopamine Receptor Expression and Distribution Dynamically Change in the Rat Nucleus Accumbens After Withdrawal From Cocaine Self-Administration. Neuroscience 169:182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo AJ, Cahill ME, Li S, Goldszer I, Henteleff R, Vanleeuwen JE, Rafalovich I, Gao R, Stachowski EK, Sampson AR, et al. (2012) Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: its role in dendritic pathology. Neurobiol Dis 45:796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. (2011) Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci 31:1895–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano S, D'Antoni A, Orban PC, Valjent E, Putignano E, Vara H, Pizzorusso T, Giustetto M, Yoon B, Soloway P, et al. (2009) Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry 66:758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. (2009) In vivo cocaine experience generates silent synapses. Neuron 63:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. (2000) Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem 275:19324–19333 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, et al. (2008) New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol 11:425–438 [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. (2008) Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 200:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park BH, Lee JH, Park SK, Kim JH. (2011) Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry 69:1026–1034 [DOI] [PubMed] [Google Scholar]
- Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. (2009) Methylphenidate-induced dendritic spine formation and ΔFosB expression in nucleus accumbens. Proc Natl Acad Sci USA 106:2915–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. (2010a) Synaptic plasticity, a symphony in GEF. ACS Chem Neurosci 1:348–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Lemtiri-Chlieh F, Levine ES, Mains RE, Eipper BA. (2011) Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J Neurosci 31:12554–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. (2010b) Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry 68:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. (2003) Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci USA 100:10523–10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. (2009) Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci 29:12275–12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. (2006) Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA 103:3399–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Zhao L, Kiraly DD, Eipper BA, Mains RE, Levine ES. (2011) Kalirin-7 is necessary for normal nMDA receptor-dependent synaptic plasticity. BMC Neurosci 12:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69:650–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM. (2010) Kalirin-7 is a key player in the formation of excitatory synapses in hippocampal neurons. ScientificWorldJournal 10:1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang Y, Eipper BA, Mains RE. (2003) Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci 23:10593–10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Kim EJ, Zhu Q, Kuchel GA, Mains RE, Eipper BA. (2011) Kalirin-7, an important component of excitatory synapses, is regulated by estradiol in hippocampal neurons. Hippocampus 21:661–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. (2001) Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol 429:388–402 [DOI] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. (2008a) Kalirin-7 is required for synaptic structure and function. J Neurosci 28:12368–12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. (2008b) Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci 28:711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Kiraly DD, Eipper-Mains JE, Ma XM, Eipper BA. (2011) Kalrn promoter usage and isoform expression respond to chronic cocaine exposure. BMC Neurosci 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Staley JK. (1999) D3 dopamine and κ opioid receptor alterations in human brain of cocaine-overdose victims. Ann NY Acad Sci 877:507–522 [DOI] [PubMed] [Google Scholar]
- May V, Schiller MR, Eipper BA, Mains RE. (2002) Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci 22:6980–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LL, Drago J, Chamberlain PM, Donovan D, Uhl GR. (1995) Retained cocaine conditioned place preference in D1 receptor deficient mice. Neuroreport 6:2314–2316 [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. (2008) Review. Transcriptional mechanisms of addiction: role of ΔFosB. Philos Trans R Soc Lond B Biol Sci 363:3245–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. (2009) A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci 29:4846–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. (2008) Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav 89:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. (2001) The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron 29:229–242 [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. (2008) Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59:621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner CA, Mains RE, Eipper BA. (2005) Kalirin: a dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist 11:148–160 [DOI] [PubMed] [Google Scholar]
- Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. (2010) Dopamine D1 and N-methyl-d-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience 168:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Peselow E. (2009) Pharmacotherapy of addictive disorders. Clin Neuropharmacol 32:277–289 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180 [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. (2007) Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci 26:749–756 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. (2010) Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann NY Acad Sci 1187:35–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. (2009) Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 29:2876–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Wu X, Wei C, Yang M, Liu Z, Ren W. (2011) Effects of NR2A and NR2B-containing N-methyl-d-aspartate receptors on neuronal-firing properties. Neuroreport 22:762–766 [DOI] [PubMed] [Google Scholar]
- Smith MJ, Thirthalli J, Abdallah AB, Murray RM, Cottler LB. (2009) Prevalence of psychotic symptoms in substance users: a comparison across substances. Compr Psychiatry 50:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. (2008) Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol 75:112–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. (2005) The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45:525–538 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587 [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA 102:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. (2012) Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct 217:337–351 [DOI] [PubMed] [Google Scholar]
- Wechsler A, Teichberg VI. (1998) Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J 17:3931–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter M, Vallone D, Samad TA, Meziane H, Usiello A, Borrelli E. (2007) Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA 104:6840–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. (1997) Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature 385:439–442 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu M. (2001) Toward a molecular understanding of psychostimulant actions using genetically engineered dopamine receptor knockout mice as model systems. J Addict Dis 20:7–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.