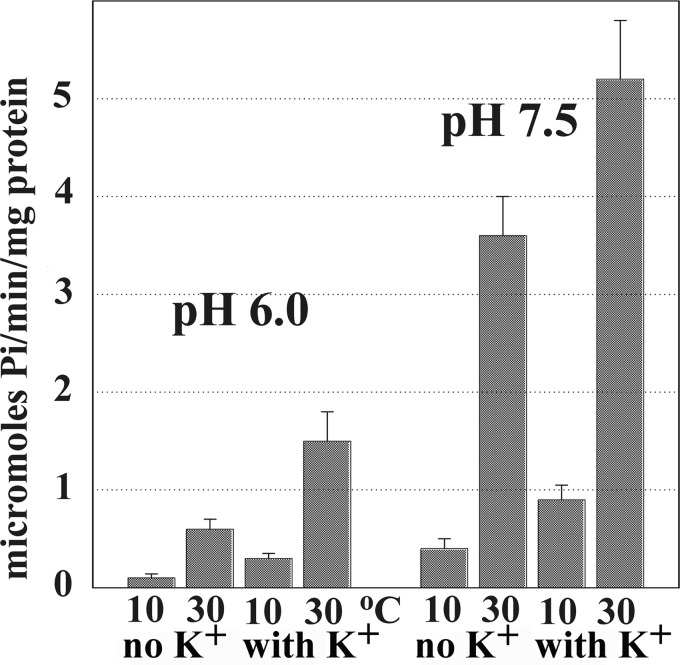
FIGURE 2.
Steady state Ca2+ ATPase activity of native Ca2+ ATPase. ATP hydrolysis was measured at pH 6.0 or 7.5 in the presence of a Ca2+ ionophore (A23187) to prevent Ca2+ accumulation in SR vesicles and ATPase “back inhibition.” The reaction mixture contained 50 mm MES (pH 6.0) or HEPES (pH 7.5), 80 mm KCl (when indicated), 3 mm MgCl2, 20 μm CaCl2, 30 μg of SR protein/ml, and 1 μm A23187. A low rate of Ca2+-independent ATPase activity, measured in the presence of EGTA and no added Ca2+, was subtracted from the total. The reaction was started by the addition of 2.5 mm ATP at 10 or 30 °C temperature, and samples were collected at time intervals. ATPase velocity was calculated from linear slopes of Pi production.