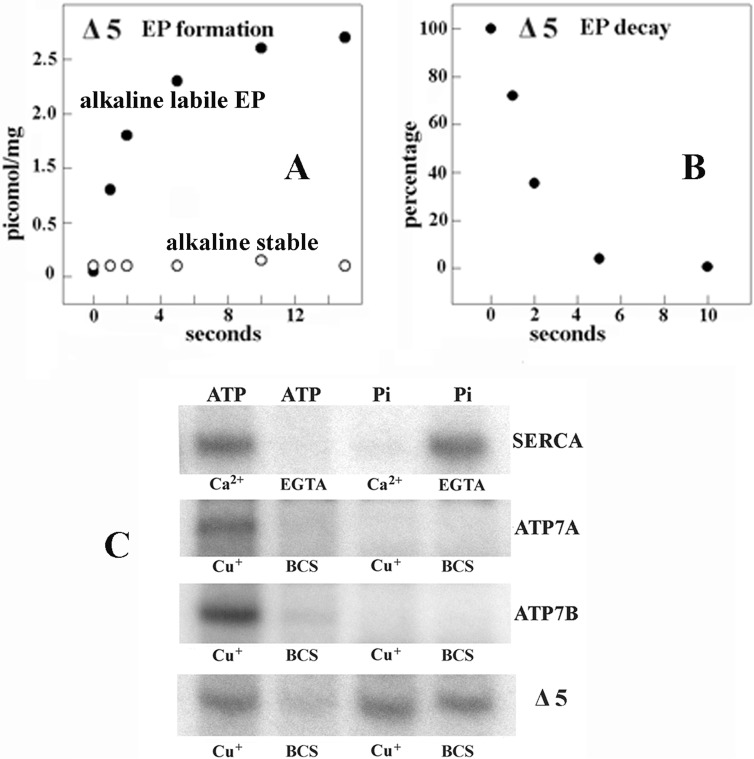
FIGURE 8.
Functional behavior of Δ5 ATP7B (large NMBD segment deleted). A and B, formation of [γ-32P]phosphoenzyme after [γ-32P]ATP utilization by Δ5 ATP7B and decay of [γ-32P]phosphoenzyme after a chase with excess nonradioactive ATP. The experiments were performed at pH 6.0 and 10 °C temperature as described in the legend to Fig. 4. The low phosphoenzyme levels are due to low concentration of expressed protein in the microsomes of the infected COS-1 cells. C, electrophoretic demonstration of phosphoenzyme formation through utilization of [γ-32P]ATP or [32P]Pi by SERCA, ATP7A, ATP7B, and Δ5 ATP7B is shown. Microsomes derived from COS1 cells expressing SERCA were incubated with 50 μm [γ-32P]ATP for 5 s at 30 °C in a reaction mixture containing 50 mm MES (pH 6.0), 3 mm MgCl2, 100 mm KCl, and 10 μm CaCl2 or 2 mm EGTA as indicated. Microsomes derived from COS1 cells expressing ATP7A, ATP7B, or Δ5 ATP7B mutant protein were incubated with 50 μm [γ-32P]ATP for 5 s at 30 °C in a reaction mixture containing 50 mm MES, pH 6.0, 300 mm KCl, 10 mm DTT, 3 mm MgCl2, and 5 μm CuCl2 or 2 mm BCS as indicated. Alternatively, microsomes derived from COS1 cells expressing SERCA were incubated with 50 μm [32P]Pi for 2 min at 30 °C in a reaction mixture containing 50 mm MES, pH 6.0, 10 mm MgCl2, 20% Me2SO4, and 1 mm CaCl2 or 2 mm EGTA as indicated. Microsomes derived from COS1 cells expressing ATP7A, ATP7B, or Δ5 ATP7B mutant protein were incubated with 50 μm [γ-32P]Pi for 2 min at 30 °C in a reaction mixture containing 50 mm MES (pH 6.0), 10 mm DTT, 10 mm MgCl2, 20% Me2SO4, and 5 μm CuCl2 or 2 mm BCS as indicated. The reactions were acid-quenched, and the samples were processed for determination of radioactive protein by electrophoretic analysis and detection of radioactivity. It is noteworthy that no kinase-mediated Ser/Thr phosphorylation was noted when Pi rather than ATP was used as a substrate.