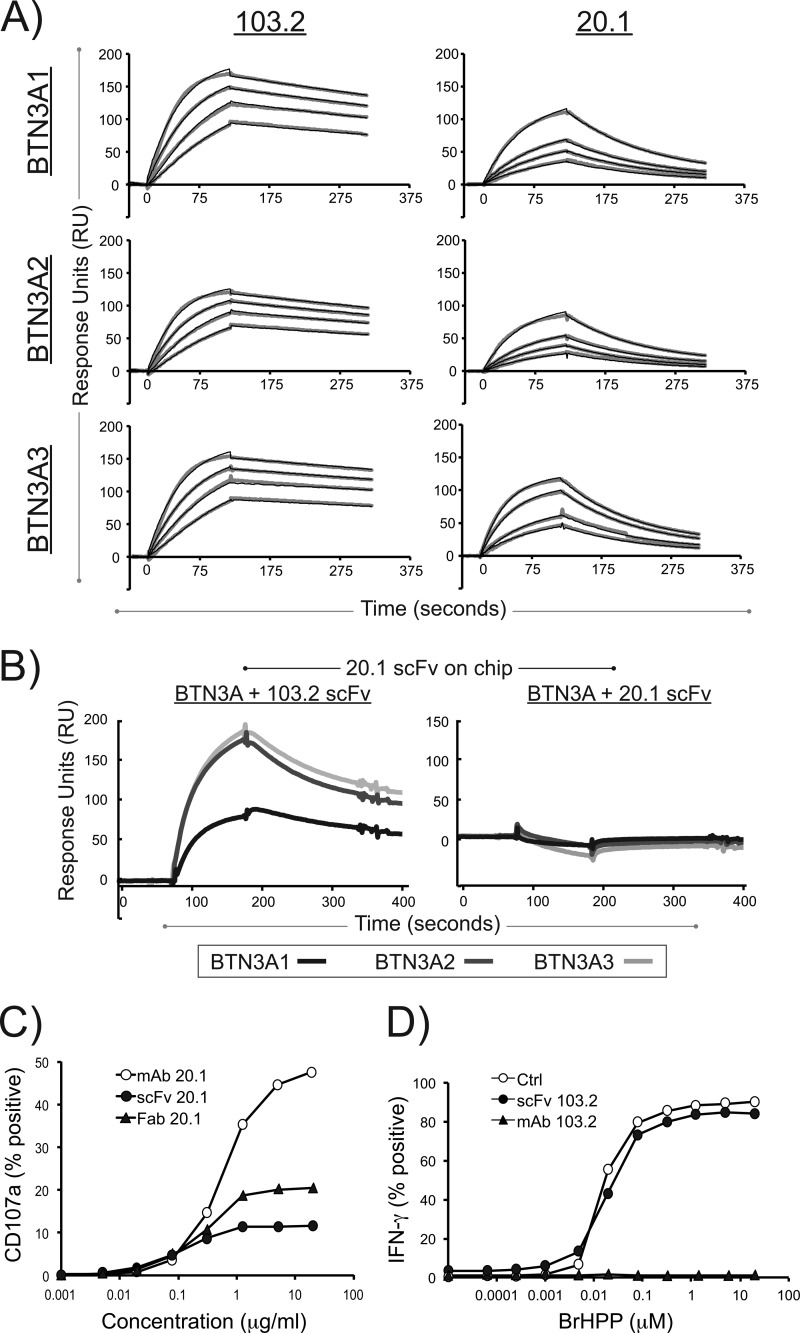
FIGURE 4.
Surface plasmon resonance of the 20. 1 and 103.2 antibodies with BTN3A1, BTN3A2, and BTN3A3 reveal high affinities and noncompetitive binding. A, representative sensograms are shown for the surface plasmon resonance analysis of 20.1 scFv and 103.2 scFv binding to the three BTN3A isoforms. Each BTN3A isoform was immobilized on an individual flow cell on a sensor chip, and the scFv were flowed as analyte at concentrations ranging from 10 to 80 nm. The data curves are shown in gray, and the modeled fit is shown as black lines. B, competition assay between the 20.1 and 103.2 scFv for BTN3A isoforms. The 20.1 scFv was immobilized on the sensor chip surface and complexes of either BTN3A-103.2scFv (left panel) or BTN3A-20.1 scFv (right panel) were flowed as analyte. Clear binding is observed with the BTN3A-103.2 scFv complex, whereas binding is blocked with the BTN3A-20.1 scFv, indicating that the two scFvs bind different epitopes on the BTN3A molecules. C, CD107a expression on the human Vγ9Vδ2 T cell line GUI following incubation with either 20.1 full-length antibody (open circles), 20.1 Fab fragments (filled triangles), or 20.1 scFv (filled circles) used at the indicated concentrations. The data are presented as the percentages of CD107a+ γδ T cells and are representative of more than three experiments. D, IFN-γ expression in human Vγ9Vδ2 T cell line GUI following activation by increasing doses of bromohydrin pyrophosphate/phosphostim (BrHPP) in the presence of the 103.2 full-length antibody (filled triangles), 103.2 scFv (filled circles), or no antibody (open circles). The data are presented as the percentages of IFN-γ+ γδ T cells and are representative of more than three experiments.