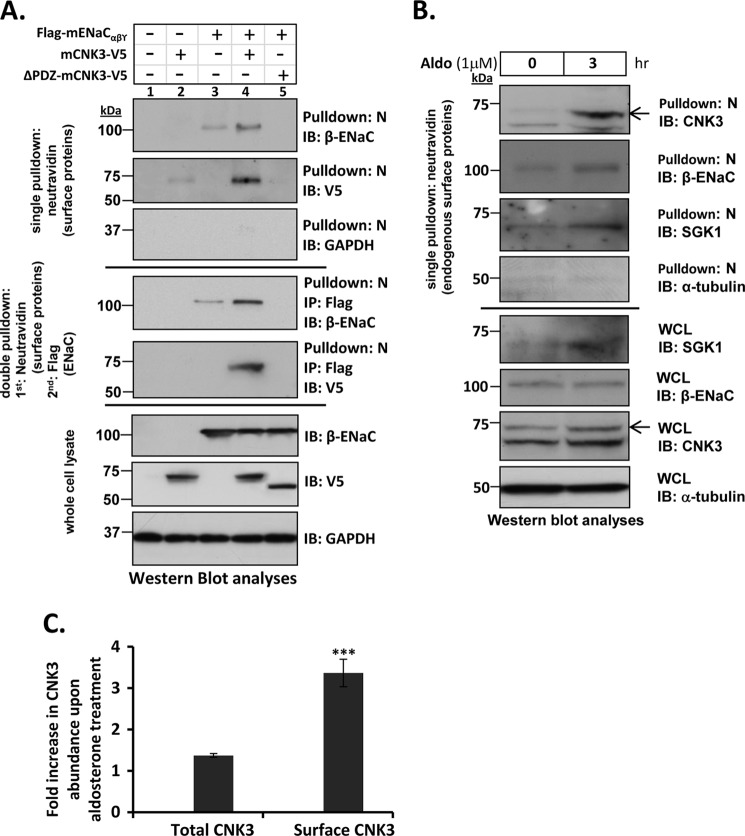
FIGURE 7.
CNK3 interacts with ENaC at the cell surface and significantly stimulates its plasma membrane abundance. A, cell surface biotinylation assays in HEK 293T cells showing a marked reduction in the ability of ΔPDZ-CNK3 to stimulate surface expression of ENaC in contrast to that of WT-CNK3. Also shown is the dependence of CNK3 on ENaC for its surface recruitment. GAPDH served as a negative control for NeutrAvidin IP (“N”) blots that primarily contained surface proteins (n = 4). Sequential cell surface biotinylation and co-immunoprecipitation assays confirm that WT-CNK3 interacts with cell surface ENaC and stimulates its plasma membrane abundance (see “Experimental Procedures” for experimental details). Blots showing total cellular expression of the various proteins specified are also depicted. In whole-cell lysates (WCL), GAPDH was used as a loading control (n = 4). B, cell surface biotinylation assays for endogenous ENaC, CNK3, and SGK1 proteins in mpkCCDc14 kidney epithelial cells. Aldosterone stimulation (1 μm for 3 h) markedly augments co-localization of ENaC and associated CNK3 and SGK1 proteins on the cell surface. Blots showing total cellular expression of the various proteins specified are also depicted. In whole-cell lysates, α-tubulin was used as a loading control (n = 4). C, graphical representation of the -fold increase in total (left bar) versus plasma membrane-associated (right bar) CNK3 in response to 3-h treatment with aldosterone. Surface expression (NeutrAvidin pulldown) and whole-cell lysate data from B were quantitated by densitometry. Bars represent signal ratios in the presence versus absence of aldosterone. Total CNK3 continued to increase at later time points, reaching a peak of ∼3-fold stimulation at 6–8 h of aldosterone treatment (not shown; see text for details). ***, p < 0.001 (n = 4). Error bars represent S.E. IB, immunoblot.