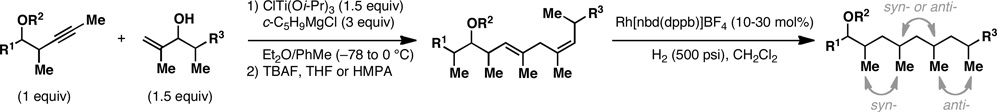
Table 1.
Deoxypropionates by metallacycle-mediated alkyne–allylic alcohol cross-coupling and directed hydrogenation.
 | ||||||
|---|---|---|---|---|---|---|
| entry | alkyne | allylic alcohol |
combined yield for coupling/deprotection (%) |
1,4-diene | yield for conversion to deoxypropionate (%) |
deoxypropionate |
| 1 | 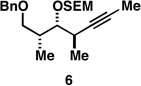 |
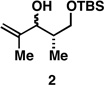 |
50a | 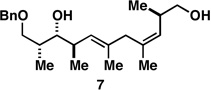 |
80c | 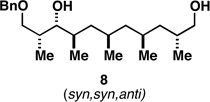 |
| 2 | 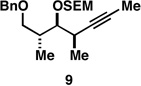 |
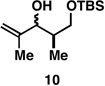 |
60a | 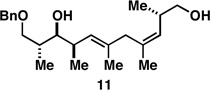 |
82c | 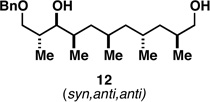 |
| 3 | 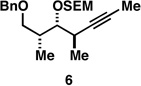 |
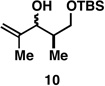 |
50a | 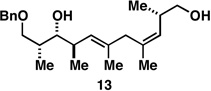 |
82c | 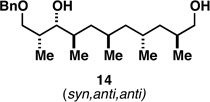 |
| 4 | 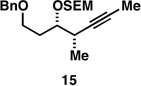 |
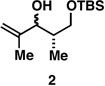 |
56a | 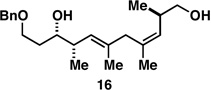 |
80c | 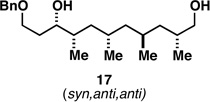 |
| 5 | 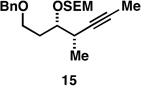 |
 |
62a | 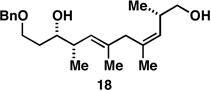 |
79c | 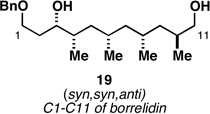 |
| 6 | 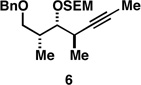 |
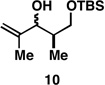 |
72b | 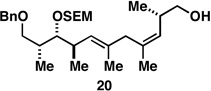 |
87c | 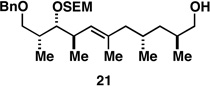 |
Reaction conditions:
For Ti-mediated reductive cross-coupling: ClTi(Oi-Pr)3, c-C5H9MgCl, PhMe, Et2O; For global desilylation: TBAF, HMPA, 35 °C.
Mono desilylation: TBAF, THF.
Rh[nbd(dppb)]BF4, CH2Cl2, H2 (500 psi) – reactions were typically run using 30 mol% of the Rh-catalyst; similar yields were observed when catalyst loading was reduced to 10 mol%. See Supporting Information for experimental details.
