Abstract
Aim/Hypothesis
Rat pancreatic islet cell apoptosis is minimal after prolonged culture in 10 mmol/l glucose (G10), largely increased in 5 mmol/l glucose (G5) and moderately increased in 30 mmol/l glucose (G30). This glucose-dependent asymmetric V-shaped profile is preceded by parallel changes in the mRNA levels of oxidative stress-response genes like Metallothionein 1a (Mt1a). In this study, we tested the effect of ZnCl2, a potent inducer of Mt1a, on apoptosis, mitochondrial oxidative stress and alterations of glucose-induced insulin secretion (GSIS) induced by prolonged exposure to low and high vs. intermediate glucose concentrations.
Methods
Male Wistar rat islets were cultured in RPMI medium. Islet gene mRNA levels were measured by RTq-PCR. Apoptosis was quantified by measuring islet cytosolic histone-associated DNA fragments and the percentage of TUNEL-positive β-cells. Mitochondrial thiol oxidation was measured in rat islet cell clusters expressing “redox sensitive GFP” targeted to the mitochondria (mt-roGFP1). Insulin secretion was measured by RIA.
Results
As observed for Mt1a mRNA levels, β-cell apoptosis and loss of GSIS, culture in either G5 or G30 vs. G10 significantly increased mt-roGFP1 oxidation. While TPEN decreased Mt1a/2a mRNA induction by G5, addition of 50–100 µM ZnCl2 to the culture medium strongly increased Mt1a/2a mRNA and protein levels, reduced early mt-roGFP oxidation and significantly decreased late β-cell apoptosis after prolonged culture in G5 or G30 vs. G10. It did not, however, prevent the loss of GSIS under these culture conditions.
Conclusion
ZnCl2 reduces mitochondrial oxidative stress and improves rat β-cell survival during culture in the presence of low and high vs. intermediate glucose concentrations without improving their acute GSIS.
Introduction
Type 2 diabetes results from the combination of insulin resistance and defective glucose stimulation of insulin secretion by the endocrine pancreas. The latter defect is due to a reduction in pancreatic β-cell mass and function [1], [2] that has been diversely attributed to low grade inflammation, mitochondrial oxidative stress or endoplasmic reticulum stress [3]. In this context, we and others have previously shown that, after prolonged culture in the presence of a large range of glucose concentrations, rat islet cell apoptosis follows an asymmetric V-shaped profile with a minimum in 10 mmol/l, a large increase in 5 mmol/l and a moderate increase in 30 mmol/l glucose [4], [5]. These changes were preceded by parallel changes in the mRNA levels of oxidative stress-response genes such as metallothionein 1a (Mt1a), heme oxygenase 1 (Hmox1) and c-Myc, suggesting a possible link between early β-cell oxidative stress and their subsequent apoptosis during prolonged culture in either low or high vs. intermediate glucose concentrations [4]. To date, despite considerable debate about whether glucose reduces or increases β-cell oxidative stress [6]–[11], a V-shaped glucose-response curve for β-cell oxidative stress has not been reported yet.
Metallothioneins are small proteins of ∼60 AA of which ∼20 are cysteine residues. Their gene expression is mainly regulated by metal transcription factors (MTF) that are activated by heavy metals and by a rise in free intracellular Zn2+ concentration ([Zn2+]i) [12]. Metallothioneins play an essential role in Zn2+ homeostasis by having the capacity to bind up to seven Zn2+ ions [13] and by controlling Zn2+ distribution to various Zn2+-binding proteins [14]. These functions are clearly regulated by changes in cellular redox state. Thus, while Zn2+ is preferentially bound to metallothioneins under reductive conditions, oxidation of their cysteine residues releases Zn2+ that can activate MTF, thereby increasing metallothionein expression and restoring cell Zn2+ buffering capacity [15]. In addition, the increase in [Zn2+]i has also been proposed to exert antioxidant and antiapoptotic effects through various mechanisms, e.g. caspase inhibition, xanthine oxidase inhibition, increased cytosolic superoxide dismutase activity and metallothionein overexpression [16].
Zn2+ is an important cofactor for insulin biosynthesis and crystallization [17] and is co-secreted with insulin. A non-synonymous polymorphism in the Slc30a8 gene encoding the insulin granule Zn2+ transporter Slc30a8 (ZnT8) has been associated with the risk of developing type 2 diabetes [18]. Interestingly, type 2 diabetic patients display a marked decrease in total plasma Zn2+ concentration together with hyperzincuria, and Zn2+ supplementation has been shown to ameliorate glycemic control in both type 1 and type 2 diabetes (reviewed in [19], [20]. A high-Zn2+ diet also improved blood glucose levels after islets transplantation in diabetic rats [21]. However, the mechanisms underlying these beneficial effects of Zn2+ have been poorly elucidated.
We therefore tested the effect of ZnCl2, a potent inducer of metallothionein expression, on islet cell apoptosis and the alterations of glucose-stimulated insulin secretion (GSIS) after prolonged culture of rat islets in low and high vs. intermediate glucose concentrations. Using the mitochondria-targeted redox-sensitive ratiometric fluorescent probe mt-roGFP1, we also tested the effect of culture in the presence of low, intermediate and high glucose concentrations with or without ZnCl2 on β-cell mitochondrial thiol oxidation state.
Materials and Methods
Materials
Dithiothreitol (DTT) and N,N,N′N′-tetrakis(-)[2-pyridylmethyl]-ethylenediamine (TPEN) were purchased from Sigma (St-Louis, MO, USA). Hydrogen peroxide (H2O2) and ZnCl2 were obtained from Acros Organics (Thermo Fisher Scientific, New Jersey, USA). Other reagents of analytical grade were purchased from Merck (Darmstadt, Germany).
Islet Isolation and Culture
Pancreatic islets were isolated from ∼200 g male Wistar rats as described [4]. Except for experiments on cell clusters, the islets were precultured for one week at 37°C and 5% CO2 in serum-free RPMI 1640 (Invitrogen, Carlsbad, CA, USA) containing 5 g/l BSA and 10 mmol/l glucose. They were then cultured for up to 1 week in the same medium containing 5, 10 or 30 mmol/l glucose (G5, G10, or G30) and various test substances, and processed for further analysis. Experimental procedures were approved by the local ethics committee for animal experimentation.
Real-time PCR
Islet gene mRNA levels were measured as described [22]. Briefly, islet total RNA was extracted using Tripure (Roche Diagnostics GmbH, Mannheim, Germany) and reversed transcribed into cDNA using 50 ng of randoms hexamers and 200 units of the enzyme RevertAid™ H Minus M-MuLV Reverse Transcriptase (Fermentas GmbH, St.Leon-Rot, Germany). Real-time PCR was performed with an iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules,CA). Primers sequences and reactions conditions are shown in Table S1. Islet gene mRNA to TATA-box binding protein (Tbp) mRNA or cyclophilin mRNA ratios (2−ΔCt) were expressed relative to the ratio in islets cultured in G10.
Immunodetection of MT1a/2a
Islets were fixed in 4% paraformaldehyde and embedded in paraffin. Five µm-thick sections were incubated overnight with a mouse anti-MT1a/2a antibody (Abcam, Cambridge, UK) diluted 1∶100, washed in Tris-buffered saline and incubated for 1 h with Alexa Red 594-conjugated goat anti-mouse IgG (Invitrogen, CA, USA) diluted 1∶200. On the second day, washed sections were incubated overnight with guinea-pig anti-insulin antibody (Invitrogen) diluted 1∶2000. On the third day, sections were washed and incubated for 1 h with Alexa Green 488-conjugated goat anti-guinea-pig antibody diluted 1∶200. Sections were then mounted with Vectashield-mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and visualized on a fluorescence microscope (FluoArc mounted on an Axioskop 40 microscope coupled to an HBO 100 camera; Carl Zeiss, Oberkochen, Germany) under standardized conditions (excitation/emission wavelengths: insulin: 475/540 nm; DAPI: 350/460 nm; MT1a/2a: 590/617 nm).
Islet Cell Apoptosis
Cytoplasmic histone-associated DNA fragments were measured on batches of 50 to 60 islets using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics) as described [22]. The percentage of apoptotic β-cells (TUNEL-positive and insulin-positive cells) was determined on 5 µm-thick islet sections using the In Situ Cell Death Detection Kit - POD (Roche Diagnostics) following the manufacturer’s instructions. Sections were then incubated overnight with a guinea-pig anti-insulin antibody (1/1000) (Invitrogen, Eugene, Oregon, USA) and incubated for 1 h with an AlexaFluor 594-conjugated anti-guinea-pig antibody (1/1000) (Invitrogen, Camarillo, CA93012, USA). Islet cell nuclei were stained with DAPI. The percentage of apoptotic β-cells was determined by manually counting TUNEL, DAPI and insulin-positive nuclei on digital images obtained by fluorescence microscopy (FluoArc mounted on an Axioskop 40 coupled to a HBO 100 camera; Carl Zeiss, Oberkochen, Germany) under standardized conditions (excitation/emission wavelengths: fluorescein: 475/540 nm; DAPI: 350/460 nm; insulin: 590/617 nm).
Adenovirus
Adenovirus encoding mt-roGFP1 under the control of the CMV promoter were generated and amplified using the pAdEasy system (Stratagene, La Jolla, CA), as previously described [11]. After purification on a gradient of CsCl, the infectious titre of viral stocks was determined with the Adeno-X™ Rapid Titer kit (Clontech, Mountain View, CA, USA).
Mitochondrial Oxidative Stress Measurement
Mt-roGFP1, which measures the thiol/disulfide equilibrium in the mitochondrial matrix, was used as an indicator of mitochondrial redox status [23], [24]. After isolation, rat islets were dispersed in clusters using trypsin and gentle pipetting in a Ca2+-free medium. Cells clusters were plated on glass coverslips and cultured overnight in RPMI 1640 medium containing G10 and 10% Fetal Bovine Serum (FBS). Cells were infected for 48 h with adenovirus coding mt-roGFP1 (multiplicity of infection ∼25 to 50) and the medium was changed for the last 18 to 24 h with a medium containing G5 or G10 and FBS with or without 50 µM ZnCl2. Mt-roGFP1 fluorescence (excitation : 405 and 480 nm, emission : 535 nm, 40X objective) was measured every 30 s in cell clusters perifused with a bicarbonate-buffered Krebs solution containing (mmol/l) NaCl (120), KCl (4.8), CaCl2 (2.5), MgCl2 (1.2), NaHCO3 (24), 1 g/l BSA (fraction V, Roche, Basel, Switzerland) and continuously gassed with O2/CO2 (94/6) to maintain pH ∼7.4. For the first 20 min, cells were perifused with the same glucose concentration as during the last period of culture but without ZnCl2. They were then perifused with 10 mmol/l DTT for 15 min to maximally reduce the probe, followed by 1 mmol/l H2O2 for 25 min to maximally oxidize mt-roGFP1. The ratio of fluorescence intensities (exc 405/480) were computed and expressed as a percentage of the difference between the mean ratio measured from 4 to 8 min after addition of DTT (set at 0%) and that measured from 14 to 18 min after addition of H2O2 (set at 100%).
Insulin Secretion
After culture, batches of 5 islets were incubated for 45 min in a bicarbonate-buffered Krebs solution containing 0.5 mmol/l glucose. Islets were then incubated for 1 h in the presence of various glucose concentrations. Insulin concentration in the medium was measured by RIA using rat insulin as a standard [25], and normalized for variations in islet DNA content measured by fluorimetry using SYBR Green I [26].
Statistical Analysis
Results are means ± SEM for the indicated number of experiments. Statistical significance of differences between groups was assessed by one-way ANOVA followed by a test of Newman-Keuls or by two-way ANOVA followed by a test of Bonferroni, as indicated in the legends. Differences were considered significant if P<0.05.
Results
Effects of Glucose and Zn2+ Chelation on Mt1a, Mt2a, ZnT1 and Znt8 mRNA Expression in Overnight Cultured Rat Islets
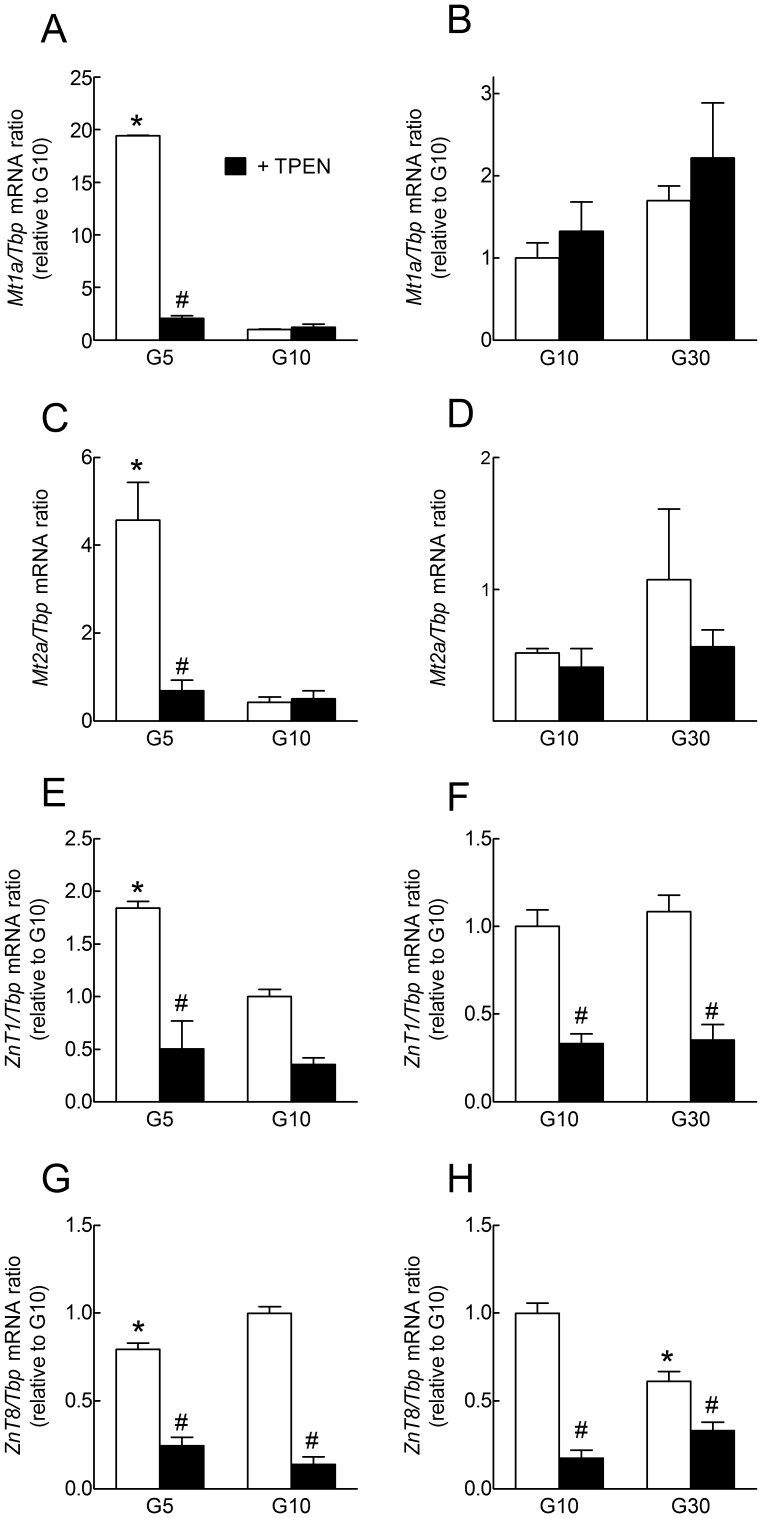
After overnight culture of whole rat islets in the presence of 5, 10 or 30 mmol/l glucose (G5, G10 or G30), islet gene mRNA levels for the typical MTF-target genes Mt1a and Mt2a were minimal in G10, largely increased in G5, and slightly but not significantly increased in G30 (Fig. 1A–D). In comparison, the mRNA levels of the other MTF-target gene Slc30a1 (Znt1) only increased ∼2-fold in G5 vs. G10 and were not affected by G30 (Fig. 1EF). In contrast, Slc30a8 (ZnT8) mRNA expression decreased after culture in either G5 or G30 vs. G10 (Fig. 1GH). Under these conditions, the membrane-permeable Zn2+ chelator TPEN almost fully inhibited the stimulation of Mt1a, Mt2a mRNA expression by culture in G5, suggesting that a rise in [Zn2+]i is involved in this effect of low glucose. In contrast, TPEN failed to affect Mt1a and Mt2a mRNA levels in G10 and G30 but significantly reduced Znt1 and ZnT8 mRNA levels under all conditions. These effects of TPEN were, however, accompanied by a ∼2 to 4-fold reduction in the mRNA levels of the housekeeping genes Tbp and cyclophilin under all culture conditions, and by a clear increase in islet cell apoptosis (not shown).
Figure 1. Effects of glucose and Zn2+ chelation by TPEN on Mt1a, Mt2a, ZnT1 and ZnT8 mRNA expression in overnight cultured rat islets.
Rat islets were precultured for 1 week in serum-free RPMI medium containing G10 and 5 g/l BSA. They were then cultured for 18 h in the presence of 5, 10 or 30 mmol/l glucose (G5, G10 or G30) with DMSO (open bars) or with 6.25 µmol/l TPEN (dissolved in DMSO) (closed bars). Mt1a, Mt2a, ZnT1 and ZnT8 to Tbp mRNA ratios were measured by real-time PCR. Except for Mt2a, the Gene to Tbp mRNA ratios were expressed relative to the ratio measured in islets cultured in G10 without TPEN. C, the mean Ct for Tbp was 27.5 in G5, 29.3 in G5+TPEN, 28 in G10 and 28.9 in G10+TPEN. D, the mean Ct for Tbp was 27.9 in G10, 28.8 in G10+TPEN, 27.8 in G30 and 29.2 in G30+TPEN. Results are means ± SEM for 3 experiments. *, P<0.05 for the effect of G5 vs. G10 and #, P<0.05 for the effect of TPEN by two-way ANOVA followed by a test of Bonferroni.
Long-term Effects of Glucose and ZnCl2 on Mt1a, Mt2a, Znt1 and ZnT8 mRNA Expression in Cultured Rat Islets
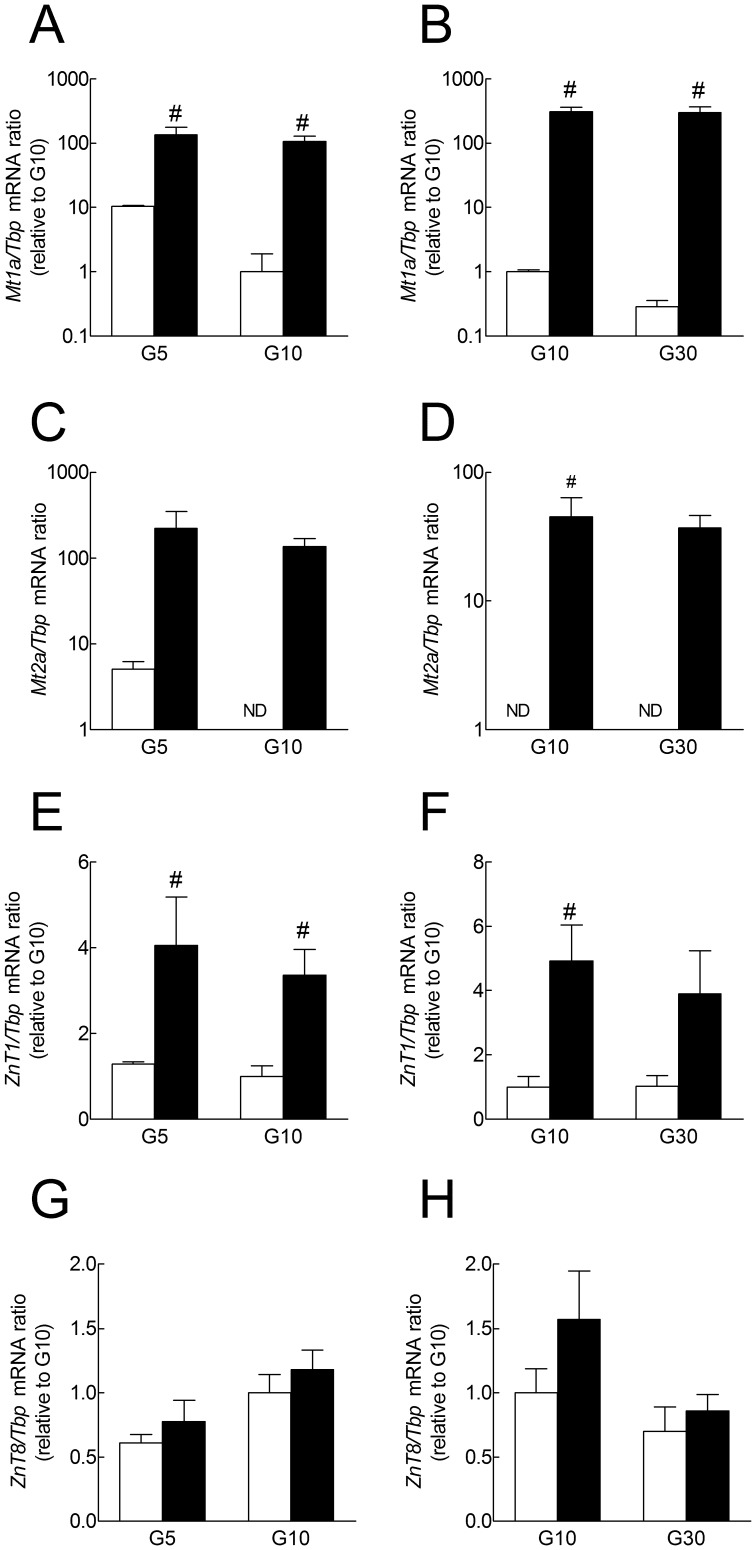
After one week of culture in G5, Mt1a mRNA expression increased ∼10 fold in comparison with islets cultured in G10 (Fig. 2A). However, contrasting with data obtained after 18 h of culture, Mt1a mRNA expression did not increase and even tended to decrease after one week of culture in G30 vs. G10 (Fig. 2B). Similar results were obtained for Mt2a while Znt1 and ZnT8 mRNA levels were not affected by one week of culture at different glucose concentrations (Fig. 2C–H). Under these conditions, addition of 100 µmol/l ZnCl2 to the medium increased Mt1a, Mt2a and Znt1 but not ZnT8 mRNA expression at all glucose concentrations, Mt1a and Mt2a mRNA reaching levels at least a 100-fold higher than in islets cultured in G10 (Fig. 2). In comparison, addition of 10 or 30 µmol/l ZnCl2 to G5 only slightly increased Mt1a mRNA expression 1.9±0.4 and 2.2±0.6 times respectively (20±4 and 24±7 times the level in G10-cultured islets, n = 3). We therefore used ZnCl2 at the concentration of 100 µmol/l in all subsequent experiments carried out with whole islets.
Figure 2. Long term effects of glucose and ZnCl2 on Mt1a, Mt2a, ZnT1 and ZnT8 mRNA expression in cultured rat islets.
After preculture, rat islets were cultured for 1 week in the presence of G5, G10 or G30 alone (open bars) or in the presence of 100 µmol/l ZnCl2 (closed bars). Gene to Tbp mRNA ratios were measured by real-time PCR and expressed relative to the ratio measured in islets cultured in G10 without ZnCl2, except for Mt2a to Tbp mRNA ratios that are shown without normalization. ND: Not Detected (unspecific products with lower Tm were amplified at Ct>30, i.e. at Mt2a/Tbp mRNA ratio <0.25). C, the mean Ct for Tbp were 27 in G5, 27.5 in G5+ZnCl2, 27.3 in G10 and 27.3 in G10+ZnCl2. D, the mean Ct for Tbp were 28.1 in G10, 28 in G10+ZnCl2, 27.8 in G30 and 28.1 in G30+ZnCl2. Please note the logarithmic Y-scale in A-D. Results are means ± SEM for 3 or 4 experiments. #, P<0.05 for the effect of ZnCl2 by two-way ANOVA followed by a test of Bonferroni. C,D, for statistical analysis of differences between groups, Mt2a/Tbp mRNA ratio was set to 0.25 when not detected.
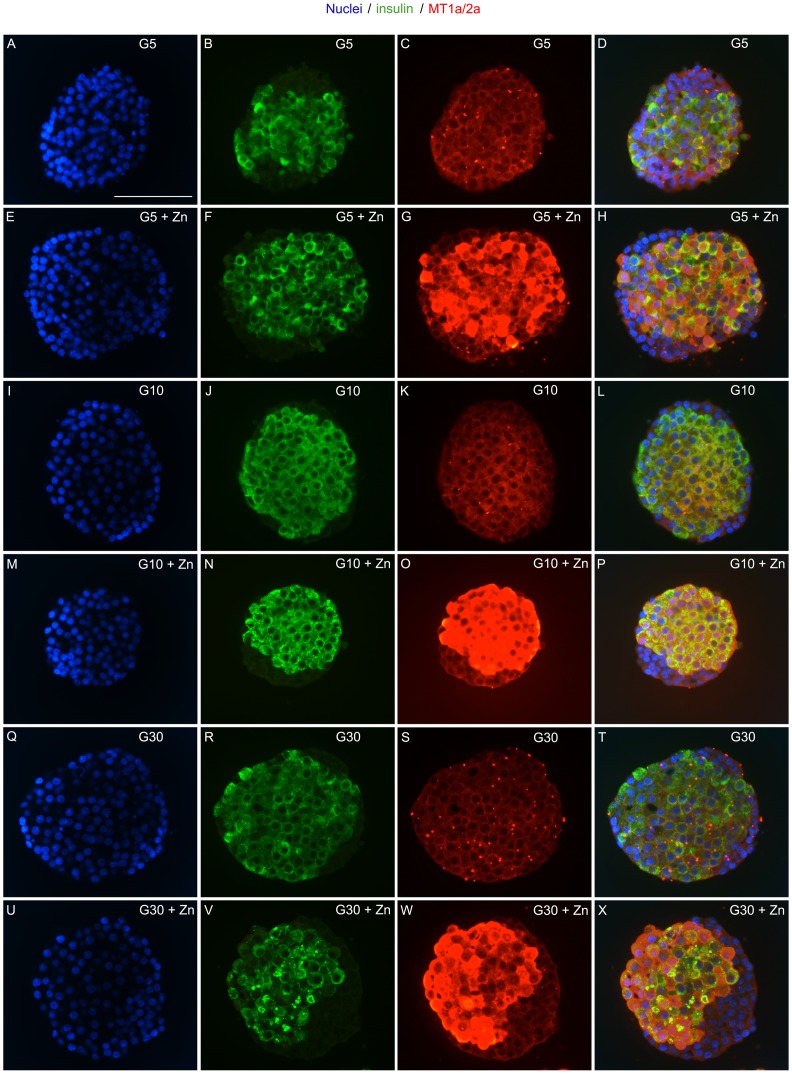
Long-term Effects of Glucose and ZnCl2 on MT1a/2a Protein Levels in Cultured Rat Islets
As shown in figure 3, MT1a/2a protein levels were not detectably affected by one week of culture in G5 or G30 vs. G10. However, addition of 100 µM ZnCl2 strongly increased MT1a/2a protein levels specifically in β-cells at all glucose concentrations.
Figure 3. Long term effects of glucose and ZnCl2 on MT1a/2a protein levels in cultured rat islets.
After preculture, rat islets were cultured for 1 week in the presence of G5, G10 or G30 alone or with 100 µmol/l ZnCl2. Islets were then fixed in paraformaldehyde solution (4%) and embedded in paraffin. Nuclei (DAPI), insulin and MT1a/2a were detected by immunohistochemistry in 5 µm-thick islets sections. Bar scale = 100 µm. A–D: islets cultured in G5; E–H : islets cultured in G5+100 µM ZnCl2 (G5+ Zn); I-L: islets cultured in G10; M-P: islets cultured in G10+100 µM ZnCl2 (G10+ Zn); Q–T: islets cultured in G30; U–X: islets cultured in G30+100 µM ZnCl2 (G30+ Zn). A,E,I,M,Q,U: DAPI staining; B,F,J,N,R,V: insulin staining; C,G,K,O,S,W: MT1a/2a staining; D,H,L,P,T,X: merge. Results are representative for 2 to 3 experiments.
Effects of ZnCl2 on Rat Islet Cell Apoptosis Induced by Prolonged Culture in Low and High Glucose
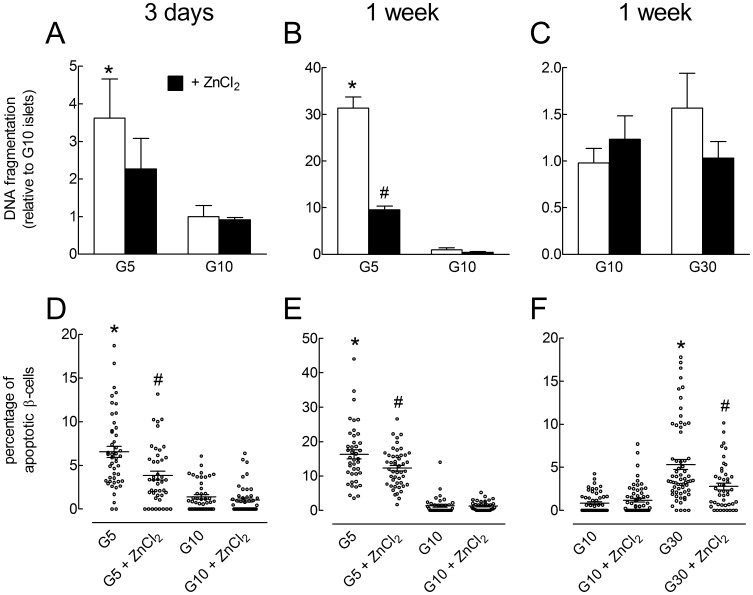
We next tested the effect of ZnCl2 on islet cell apoptosis induced by prolonged culture in extreme glucose concentrations. As shown in figure 4AD, three days of culture in G5 instead of G10 induced a ∼3.5-fold increase in cytoplasmic histone-associated DNA fragments and a ∼4.5-fold increase in the percentage of TUNEL-positive β-cell nuclei. Addition of ZnCl2 during culture significantly reduced islet cell DNA fragmentation by ∼38% and the percentage of apoptotic β-cells by ∼68%. After one week of culture in G5, DNA fragmentation increased ∼30-fold while the percentage of apoptotic β-cells increased ∼12 fold (Fig. 4BE), and addition of ZnCl2 to the medium significantly reduced these effects by 27% and 70% respectively. After one week of culture in G30, islet cytoplasmic DNA fragments only tended to increase ∼1.5 fold, but the percentage of apoptotic β-cells was ∼6 times higher than in islets cultured in G10 (Fig. 4CF). Under these conditions, ZnCl2 reduced the stimulation of DNA fragmentation by ∼90% and the increase in the proportion of TUNEL-positive β-cells by 65%. These results indicate that 100 µmol/l ZnCl2 exerts a protective effect against rat β-cell apoptosis induced by chronic exposure to extreme glucose concentrations.
Figure 4. Effects of glucose and ZnCl2 on the stimulation of rat islet cell apoptosis by prolonged culture in low and high glucose concentrations.
Rat islets were cultured for 3 days (A, D) or 1 week (B,C,E,F) in the presence of G5, G10 or G30±100 µmol/l ZnCl2 as indicated. A, B, C, DNA fragmentation was measured by an ELISA kit and expressed relative to the value measured in islets cultured in G10. D, E, F, the percentage of apoptotic β-cells was measured by TUNEL. Results are means ± SEM for 3–5 experiments. *, P<0.05 for the effect of G5 or G30 vs. G10 and #, P<0.05 for the effect of ZnCl2 by two-way ANOVA followed by a test of Bonferroni (A,B,C) or by one-way ANOVA followed by a test of Newman-Keuls (D,E,F).
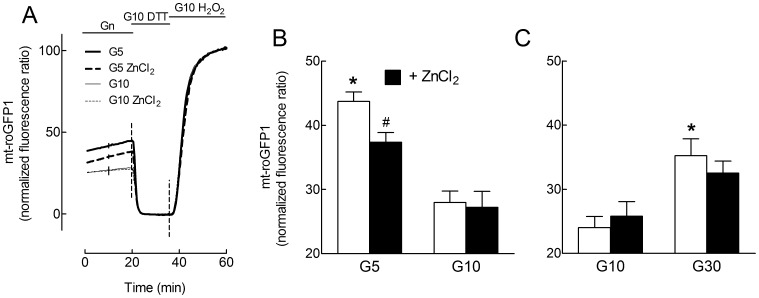
Effects of Glucose and ZnCl2 on Mitochondrial Oxidative Stress in Rat Islet Cell Clusters
As ZnCl2 has been proposed to improve cell resistance to oxidative stress, we next tested its effect on early mitochondrial oxidative stress induced by extreme glucose concentrations in rat islet cell clusters expressing mt-roGFP1. In these preparations, the concentration of ZnCl2 had to be reduced to 50 µmol/l to avoid a strong increase in mt-roGFP1 oxidation in G10-cultured clusters (data not shown). As shown in figure 5BC, overnight culture in the presence of G5 or G30 instead of G10 significantly increased mt-roGFP1 oxidation in rat islet cell clusters, reflecting an increase in thiol (e.g. glutathione) oxidation in the mitochondrial matrix. Addition of ZnCl2 to the culture medium diminished by ∼40% the level of mt-roGFP1 oxidation in G5, and tended to reduce by ∼23% that in G30. These results suggest that ZnCl2 exerts some antioxidant effect in rat islet cell clusters exposed to low and high vs. intermediate glucose concentrations.
Figure 5. Effects of glucose and ZnCl2 on mitochondrial thiol/disulfide equilibrium in cultured rat islet cell clusters.
Rat islet cell clusters infected with an adenovirus coding mt-roGFP1 were cultured overnight in the presence of G5, G10 or G30±50 µmol/l ZnCl2 as indicated. The ratio of mt-roGFP1 fluorescence intensities (exc 405/480 nm) was measured during 20 min of perfusion in a medium containing the same glucose concentration and expressed as a percentage of the difference between the mean ratio measured from 4 to 8 min after addition of 10 mmol/l DTT (set at 0%) and that measured from 14 to 18 min after addition of 1 mmol/l H2O2 (set at 100%) (B,C). Results are means ± SEM for 3 experiments (9–19 clusters). B,C, *, P<0.05 for the effect of G5 or G30 vs. G10 and #, P<0.05 for the effect of ZnCl2 by two-way ANOVA followed by a test of Bonferroni.
Effects of ZnCl2 on the Alterations of Glucose-induced Insulin Secretion after Prolonged Culture in Low and High Glucose
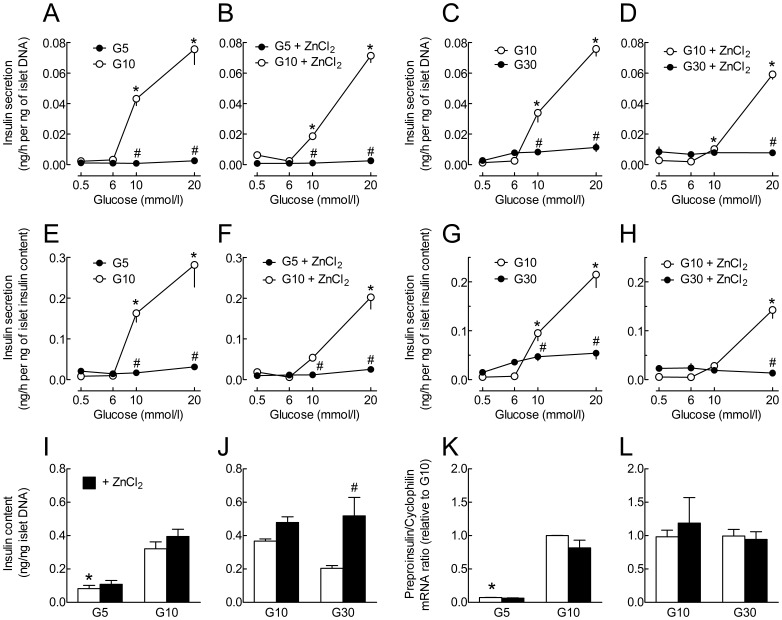
We finally tested the effects of ZnCl2 during culture on islet insulin content and acute GSIS. As shown in figure 6A,B,E,F,I, three days of culture in the presence of G5 decreased the islet insulin content by ∼75%, decreased the absolute rate of insulin secretion in G0.5 and G6, and profoundly inhibited its stimulation by G10 and G20. Addition of ZnCl2 to the culture medium did not affect the islet insulin content or GSIS of islets cultured in G5, but significantly reduced the acute insulin secretory response to G10 of islets cultured in G10. In comparison with culture in G5, one week culture in G30 only reduced the islet insulin content by ∼30% and differently affected the GSIS, with a slight increase in the rate of insulin secretion in the presence of G6, and a reduced stimulation of insulin secretion in response to G10 and G20 (Fig. 6C,D,G,H,J). Although ZnCl2 prevented the decrease in islet insulin content during culture in G30, it did not improve their GSIS. The increase in insulin content was not due to an increase in preproinsulin mRNA levels (Figure 6K,L). Thus, despite the beneficial effect of ZnCl2 on β-cell survival, ZnCl2 did not protect against the alterations of GSIS induced by culture in extreme glucose concentrations.
Figure 6. Effects of glucose and ZnCl2 during culture on subsequent glucose-stimulated insulin secretion and on preproinsulin mRNA expression.
Islets were cultured for 3 days in the presence of G5 or G10 alone or with 100 µmol/l ZnCl2 (A,B,E,F,I), or for 1 week in the presence of G10 or G30 alone or with 100 µmol/l ZnCl2 (C,D,G,H,J). Batches of 5 islets were then pre-incubated for 45 min in G0.5 in the absence of ZnCl2 and next incubated for 1 h in the presence of G0.5, G6, G10 or G20. The islet insulin (I,J) and DNA content were measured at the end of the incubation. Insulin secretion was normalized for differences in islet DNA content (A–D) or for differences in islet insulin content (E–H). *, P<0.05 for the acute effect of glucose vs. G0.5 and #, P<0.05 for the effect of culture in G5 or G30 vs. G10 by two-way ANOVA followed by a test of Bonferroni. I, J, *P<0.05 for the effect of culture in G5 and G30 vs. G10 and #, P<0.05 for the effect of ZnCl2 during culture by two-way ANOVA followed by a test of Bonferroni. K–L, after preculture, rat islets were cultured for 1 week in G5, G10 or G30 alone (open bars) or in the presence of 100 µmol/l ZnCl2 (closed bars). Preproinsulin to cyclophilin mRNA ratios were measured by real-time PCR and expressed relative to the ratio measured in islets cultured in G10. K, the mean Ct for cyclophilin were 23.7 in G5, 24 in G5+ZnCl2, 24.1 in G10 and 24.4 in G10+ZnCl2. L, the mean Ct for cyclophilin were 23.7 in G10, 23.9 in G10+ZnCl2, 23.8 in G30 and 24 in G30+ZnCl2. Results are means ± SEM for 3 or 4 experiments. *, P<0.05 for the effect of culture in G5 vs. G10 by two-way ANOVA followed by a test of Bonferroni.
Discussion
We have previously shown that prolonged culture of rat islets in the presence of low or high vs. intermediate glucose concentrations rapidly induces the expression of oxidative stress-response genes such as Mt1a and Hmox1, followed by later increase in islet cell apoptosis and marked reduction of GSIS [4]. We now provide further evidence that these changes are associated with early parallel changes in mitochondrial oxidative stress, and demonstrate that ZnCl2, which potently induces Mt1a and Mt2a expression, exerts a protective effect on mitochondrial thiol oxidation and subsequent β-cell apoptosis without improving GSIS.
In isolated rat islets cultured overnight in the presence of increasing glucose concentrations, the mRNA levels of the MTF-target genes Mt1a and Mt2a were minimal in G10, markedly increased in G5, and tended to increase in G30 vs. G10. These glucose effects were associated with parallel changes in mt-roGFP1 oxidation, a good indicator of thiol (mainly glutathione) oxidation in the mitochondrial matrix [11], [27], suggesting the presence of mitochondrial oxidative stress after 18h culture in either low or high vs. intermediate glucose concentrations. We have recently shown that mt-roGFP1 oxidation is acutely stimulated in rat islet cell clusters upon a reduction in glucose concentration from 10 to 2 mmol/l but that it is not increased upon glucose stimulation from 10 to 30 mmol/l glucose [11]. Together with the present study, these data indicate that the stimulation of oxidative stress induced by G30 is slower than that induced by G5, as is the case for the stimulation of β-cell apoptosis under these culture conditions [4]. However, in both G5 and G30, mt-roGFP1 oxidation occurred earlier than β-cell apoptosis, suggesting that the latter may result from mitochondrial oxidative stress. In that scenario, we postulate that the increase in Mt1a and Mt2a mRNA levels are sensitive indicators of this type of stress. Although we did not fully investigate the mechanism of Mt1a and Mt2a mRNA inductions, it is possible that oxidation of metallothioneins releases Mt-bound Zn2+, with consequent activation of metal transcription factor-1 (MTF-1) and increased expression of its target genes Mt1a, Mt2a and Znt1 [28], [29]. In comparison, expression of the type 2 diabetes gene Slc30a8 that encodes the β-cell specific granular zinc transporter ZnT8 was not regulated in parallel with Znt1 or Mts, in agreement with the observations that it is not induced by ZnSO4 [30] nor by ZnCl2 (the present study). Despite the large changes in Mt1a and Mt2a mRNA levels, MT1a/2a protein levels were not detectably increased by culture in low or high vs. intermediate glucose concentrations. This discordance between changes in Mt mRNA and protein levels could result from the low sensitivity of immunohistochemistry and, at least in low glucose, from a global decrease in protein translation in G5 vs. G10 [31].
It has recently been shown that the free cytosolic Zn2+ concentration ([Zn2+]c) in CD1 mouse islets decreases from ∼800 to ∼400 pmol/l after 24 h culture in 3 vs. 16.7 mmol/l glucose while Mt1-2 mRNA levels increase and ZiP6-8 expression decreases under these conditions [32]. Although these results seem to argue against a role of a rise in [Zn2+]c in the stimulation of Mt gene expression by culture in low glucose, our hypothesis is strongly supported by the observation that the membrane-permeable Zn2+ chelator TPEN fully suppressed the induction of MTF-target gene expression by G5. Thus, we interpret the late decrease in [Zn2+]c measured by Bellomo et al. after 24 h culture in 3 vs. 16.7 mmol/l glucose as a possible consequence of the increase in metallothionein expression (although it was not detected by immunohistochemistry) and Zn2+-buffering capacity under these culture conditions. Alternatively, glucose-induced changes in [Zn2+] might be different in the cytosolic and nuclear compartments of islet cells [33], or between mouse and rat β-cells.
It has previously been shown that Zn2+ supplementation reduces early graft failure in diabetic rats transplanted with syngeneic islets [21], protects mice from diabetes induced by multiple low doses of streptozotocin [34] and ameliorates glucose tolerance in type 1 and type 2 diabetic patients [19], [35], but the underlying mechanisms are not clear [20]. Also in vitro, addition of ZnSO4 to the culture medium partially protected islet cells against the toxic effect of streptozotocin [36]. In the present study, addition of 50–100 µmol/l ZnCl2 to the culture medium significantly reduced mitochondrial thiol oxidation and β-cell apoptosis triggered by prolonged exposure to low or high vs. intermediate glucose concentrations. These effects were unlikely due to the negligible increase in chloride anions but rather resulted from the provision of Zn2+, a trace element surprisingly absent from standard RPMI medium. In another study in which medium contained 5 mg/ml BSA as in ours, ∼75% of Zn2+ was bound to BSA [37]. Thus, the antiapoptotic effects of Zn2+ on whole islets were observed at a concentration similar to that measured in rodent plasma [37] but lower than those reported to exert proapoptotic effects in β-cells or other cell types [38]–[41]. Differences in Zn2+ binding or the addition of Zn2+ through FBS may explain the need to reduce ZnCl2 concentration at 50 µmol/l to avoid apoptosis of islet cell clusters. Although it has been shown that addition of 90 µmol/l ZnSO4 does not increase the intracellular zinc content of INS-1E cells [41], a recent study using a new zinc-sensitive fluorescent protein in mouse islet cell clusters has recently demonstrated that addition of 50 µmol/l ZnCl2 approximately doubled their [Zn2+]c while increasing Mt1 gene expression [32]. It is therefore likely that ZnCl2 treatment induces a rise in [Zn2+]c in rat as in mouse islets.
The beneficial effect of ZnCl2 on mitochondrial glutathione oxidation after culture in low (and to some extent high) vs. intermediate glucose concentrations may contribute to its antiapoptotic effect in β-cells. Although Zn2+ deficiency has been shown to increase oxidative stress in other cell types, few studies have demonstrated that Zn2+ decreases oxidative stress (reviewed in [42]). The mechanism could involve the increase in metallothionein expression, a group of proteins that play a role in Zn2+ distribution to the lipids and in protection of the proteins damaged by oxidative stress [15]. Mts are also known to be ROS scavengers with a capacity to capture hydroxyl radicals ∼50 times greater than that of glutathione [43]. The mechanism could also involve the role of Zn2+ in Cu/Zn superoxide dismutase (SOD1) activity, an enzyme which removes the superoxide radical, although it has been shown that Zn2+ deficiency does not decrease SOD1 activity [44], [45]. In addition, other pathways, such as the inhibition of caspase 3, 6 and 9 could play a role in the antiapoptotic effect of Zn2+ (reviewed in [42]).
Despite the significant improvement in β-cell survival, Zn2+ did not have any protective effect on the alterations of GSIS induced by prolonged culture in extreme glucose concentrations, except for a significant increase in the insulin content of high glucose-cultured islets. Actually, addition of ZnCl2 during culture even decreased subsequent insulin secretion in the presence of 10 mmol/l glucose, an effect that was more pronounced when insulin secretion was expressed relative to the islet insulin content. These effects, including the increase in insulin content after culture in 30 mmol/l glucose, could result from the inhibition of Ca2+ influx and GSIS by Zn2+ in a slowly reversible manner [37], [46], [47]. Alternatively, we cannot exclude that the inhibition of insulin secretion by ZnCl2 in G10 indirectly resulted from an inhibition of glucagon secretion by α-cells (reviewed in [48]), hence of [cAMP] in β-cells. However, a similar lack of improvement of GSIS was observed when islet cell apoptosis triggered by prolonged culture in 5 instead of 10 mmol/l glucose was inhibited by 50–70% with the SOD and catalase-mimetic manganese (III)tetrakis (4-benzoic acid)porphyrin (MnTBAP) [49]. Two hypotheses may explain these results. Either β-cell function is more sensitive than survival to the remaining level of oxidative stress present in the presence of Zn2+ or MnTBAP, or the loss of GSIS under these culture conditions is unrelated to mitochondrial oxidative stress. Thus, although an increase in β-cell mass with no alteration in secretory function may contribute to the beneficial effect of Zn2+ supplementation on glucose tolerance in diabetes, our results emphasize the importance of testing GSIS in addition to cell survival when testing potential treatments of stressed β-cells.
In conclusion, culture of rat pancreatic islets in either low or high vs. intermediate glucose concentrations triggers early mitochondrial oxidative stress with Mt1a/2a mRNA expression and late β-cell apoptosis with loss of GSIS. ZnCl2 reduces mitochondrial oxidative stress and rat β-cell apoptosis under these culture conditions without improving GSIS.
Supporting Information
Sequences of oligonucleotide primers and PCR conditions. The specificity of sense and anti-sense primers was checked by BLAST search. The thermal cycle profile consisted of a 3 min step at 95°C to release DNA polymerase activity followed by 40 cycles of amplification (15 sec denaturation step at 95°C, 45-60-90 sec annealing step at 60–62°C, and eventual 15–30 sec extension step at 80-82-84°C). Under these conditions, PCR efficiencies were ∼0.95 to 1.0. The melting temperature (Tm) of the amplicons was systematically determined at the end of the PCR to check their specificity. Their size corresponded to that expected from published sequences, as determined by agarose gel electrophoresis. *, Islet sample cDNA input in 25 µl reactions (ng total RNA equivalent).
(DOC)
Acknowledgments
We thank Denis Charlier and Fabien Knockaert for expert technical help and Yves Guiot for access to immunohistochemistry facility at the Pôle de Morphologie of the institute. We also thank Brice Maron for developing a computer program facilitating manual counting of TUNEL-positive insulin-positive cells on digitized islet sections.
Funding Statement
This work was supported by the Fonds de la Recherche Scientifique-FNRS (Belgium) (grants 3.4516.09, 1.5012.11 and 3.4521.12) and the Société Francophone du Diabète (France). JD is recipient of a fellowship from the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture, Belgium. JCJ is Research Director of the Fonds de la Recherche Scientifique-FNRS, Belgium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. (2003) β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110. [DOI] [PubMed] [Google Scholar]
- 2. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC (2008) Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 10 Suppl 432–42. [DOI] [PubMed] [Google Scholar]
- 3. van Raalte DH, Diamant M (2011) Glucolipotoxicity and beta cells in type 2 diabetes mellitus: target for durable therapy? Diabetes Res Clin Pract 93 Suppl 1S37–S46. [DOI] [PubMed] [Google Scholar]
- 4. Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC (2009) Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia 52: 463–476. [DOI] [PubMed] [Google Scholar]
- 5. Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, et al. (1998) Glucose and tolbutamide induce apoptosis in pancreatic β-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem 273: 33501–33507. [DOI] [PubMed] [Google Scholar]
- 6. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, et al. (1999) Hyperglycemia causes oxidative stress in pancreatic β-cells of GK rats, a model of type 2 diabetes. Diabetes 48: 927–932. [DOI] [PubMed] [Google Scholar]
- 7. Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, et al. (2003) Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 278: 9796–9801. [DOI] [PubMed] [Google Scholar]
- 8. Robertson RP (2004) Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J Biol Chem 279: 42351–42354. [DOI] [PubMed] [Google Scholar]
- 9. Martens GA, Cai Y, Hinke S, Stange G, Van de CM, et al. (2005) Glucose suppresses superoxide generation in metabolically responsive pancreatic beta cells. J Biol Chem 280: 20389–20396. [DOI] [PubMed] [Google Scholar]
- 10. Sarre A, Gabrielli J, Vial G, Leverve XM, Assimacopoulos-Jeannet F (2012) Reactive oxygen species are produced at low glucose and contribute to the activation of AMPK in insulin-secreting cells. Free Radic Biol Med 52: 142–150. [DOI] [PubMed] [Google Scholar]
- 11. Roma LP, Duprez J, Takahashi HK, Gilon P, Wiederkehr A, et al. (2012) Dynamic measurements of mitochondrial hydrogen peroxide concentration and glutathione redox state in rat pancreatic β-cells using ratiometric fluorescent proteins: confounding effects of pH with HyPer but not roGFP1. Biochem J 441: 971–978. [DOI] [PubMed] [Google Scholar]
- 12. Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59: 95–104. [DOI] [PubMed] [Google Scholar]
- 13. Fischer EH, Davie EW (1998) Recent excitement regarding metallothionein. Proc Natl Acad Sci U S A 95: 3333–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol 146: 443–459. [DOI] [PubMed] [Google Scholar]
- 15. Maret W (2011) Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem 16: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 16. Bosco MD, Mohanasundaram DM, Drogemuller CJ, Lang CJ, Zalewski PD, et al. (2010) Zinc and zinc transporter regulation in pancreatic islets and the potential role of zinc in islet transplantation. Rev Diabet Stud 7: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emdin SO, Dodson GG, Cutfield JM, Cutfield SM (1980) Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic B-cell. Diabetologia 19: 174–182. [DOI] [PubMed] [Google Scholar]
- 18. Cauchi S, Del GS, Choquet H, D’Aleo V, Groves CJ, et al. (2010) Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab 100: 77–82. [DOI] [PubMed] [Google Scholar]
- 19. Taylor CG (2005) Zinc, the pancreas, and diabetes: insights from rodent studies and future directions. Biometals 18: 305–312. [DOI] [PubMed] [Google Scholar]
- 20. Jansen J, Karges W, Rink L (2009) Zinc and diabetes–clinical links and molecular mechanisms. J Nutr Biochem 20: 399–417. [DOI] [PubMed] [Google Scholar]
- 21. Okamoto T, Kuroki T, Adachi T, Ono S, Hayashi T, et al. (2011) Effect of zinc on early graft failure following intraportal islet transplantation in rat recipients. Ann Transplant 16: 114–120. [DOI] [PubMed] [Google Scholar]
- 22. Duprez J, Jonas JC (2011) Role of activating transcription factor 3 in low glucose- and thapsigargin-induced apoptosis in cultured mouse islets. Biochem Biophys Res Commun 415: 294–299. [DOI] [PubMed] [Google Scholar]
- 23. Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, et al. (2004) Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279: 22284–22293. [DOI] [PubMed] [Google Scholar]
- 24. Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, et al. (2004) Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053. [DOI] [PubMed] [Google Scholar]
- 25. Heding LG (1972) Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia 8: 260–266. [DOI] [PubMed] [Google Scholar]
- 26. Leggate J, Allain R, Isaac L, Blais BW (2006) Microplate fluorescence assay for the quantification of double stranded DNA using SYBR Green I dye. Biotechnol Lett 28: 1587–1594. [DOI] [PubMed] [Google Scholar]
- 27. Meyer AJ, Dick TP (2010) Fluorescent protein-based redox probes. Antioxid Redox Signal 13: 621–650. [DOI] [PubMed] [Google Scholar]
- 28. Andrews GK (2001) Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals 14: 223–237. [DOI] [PubMed] [Google Scholar]
- 29. Samson SL, Gedamu L (1998) Molecular analyses of metallothionein gene regulation. Prog Nucleic Acid Res Mol Biol 59: 257–288. [DOI] [PubMed] [Google Scholar]
- 30. Lefebvre B, Vandewalle B, Balavoine AS, Queniat G, Moerman E, et al. (2012) Regulation and functional effects of ZnT8 in human pancreatic islets. J Endocrinol 214: 225–232. [DOI] [PubMed] [Google Scholar]
- 31. Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, et al. (2007) Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50: 1442–1452. [DOI] [PubMed] [Google Scholar]
- 32. Bellomo EA, Meur G, Rutter GA (2011) Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet beta-cells. J Biol Chem 286: 25778–25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mocchegiani E, Giacconi R, Malavolta M (2008) Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med 14: 419–428. [DOI] [PubMed] [Google Scholar]
- 34. Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H (2000) Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia 43: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 35. Gunasekara P, Hettiarachchi M, Liyanage C, Lekamwasam S (2011) Effects of zinc and multimineral vitamin supplementation on glycemic and lipid control in adult diabetes. Diabetes Metab Syndr Obes 4: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohly P, Wang Z, Abel J, Gleichmann H (1998) Zinc sulphate induced metallothionein in pancreatic islets and protected against the diabetogenic toxin streptozotocin. Talanta 46: 355–359. [DOI] [PubMed] [Google Scholar]
- 37. Ferrer R, Soria B, Dawson CM, Atwater I, Rojas E (1984) Effects of Zn2+ on glucose-induced electrical activity and insulin release from mouse pancreatic islets. Am J Physiol 246: C520–C527. [DOI] [PubMed] [Google Scholar]
- 38. Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH (1999) Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A 96: 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamatake M, Iguchi K, Hirano K, Ishida R (2000) Zinc induces mixed types of cell death, necrosis, and apoptosis, in molt-4 cells. J Biochem 128: 933–939. [DOI] [PubMed] [Google Scholar]
- 40. Untergasser G, Rumpold H, Plas E, Witkowski M, Pfister G, et al. (2000) High levels of zinc ions induce loss of mitochondrial potential and degradation of antiapoptotic Bcl-2 protein in in vitro cultivated human prostate epithelial cells. Biochem Biophys Res Commun 279: 607–614. [DOI] [PubMed] [Google Scholar]
- 41. Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, et al. (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119: 4199–4206. [DOI] [PubMed] [Google Scholar]
- 42. Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD (2001) The role of zinc in caspase activation and apoptotic cell death. Biometals 14: 315–330. [DOI] [PubMed] [Google Scholar]
- 43. Miura T, Muraoka S, Ogiso T (1997) Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci 60: L–9. [DOI] [PubMed] [Google Scholar]
- 44. Oteiza PL, Olin KL, Fraga CG, Keen CL (1996) Oxidant defense systems in testes from zinc-deficient rats. Proc Soc Exp Biol Med 213: 85–91. [DOI] [PubMed] [Google Scholar]
- 45. Parat MO, Richard MJ, Beani JC, Favier A (1997) Involvement of zinc in intracellular oxidant/antioxidant balance. Biol Trace Elem Res 60: 187–204. [DOI] [PubMed] [Google Scholar]
- 46. Ghafghazi T, McDaniel ML, Lacy PE (1981) Zinc-induced inhibition of insulin secretion from isolated rat islets of Langerhans. Diabetes 30: 341–345. [DOI] [PubMed] [Google Scholar]
- 47. Figlewicz DP, Heldt A, Forhan SE, Grodsky GM (1981) Effect of exogenous zinc on insulin secretion in vitro. Endocrinology 108: 730–732. [DOI] [PubMed] [Google Scholar]
- 48. Hardy AB, Serino AS, Wijesekara N, Chimienti F, Wheeler MB (2011) Regulation of glucagon secretion by zinc: lessons from the beta cell-specific Znt8 knockout mouse model. Diabetes Obes Metab 13 Suppl 1: 112–117. [DOI] [PubMed] [Google Scholar]
- 49. Roma LP, Pascal SM, Duprez J, Jonas JC (2012) Mitochondrial oxidative stress contributes differently to rat pancreatic islet cell apoptosis and insulin secretory defects after prolonged culture in a low non-stimulating glucose concentration. Diabetologia 55: 2226–2237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of oligonucleotide primers and PCR conditions. The specificity of sense and anti-sense primers was checked by BLAST search. The thermal cycle profile consisted of a 3 min step at 95°C to release DNA polymerase activity followed by 40 cycles of amplification (15 sec denaturation step at 95°C, 45-60-90 sec annealing step at 60–62°C, and eventual 15–30 sec extension step at 80-82-84°C). Under these conditions, PCR efficiencies were ∼0.95 to 1.0. The melting temperature (Tm) of the amplicons was systematically determined at the end of the PCR to check their specificity. Their size corresponded to that expected from published sequences, as determined by agarose gel electrophoresis. *, Islet sample cDNA input in 25 µl reactions (ng total RNA equivalent).
(DOC)