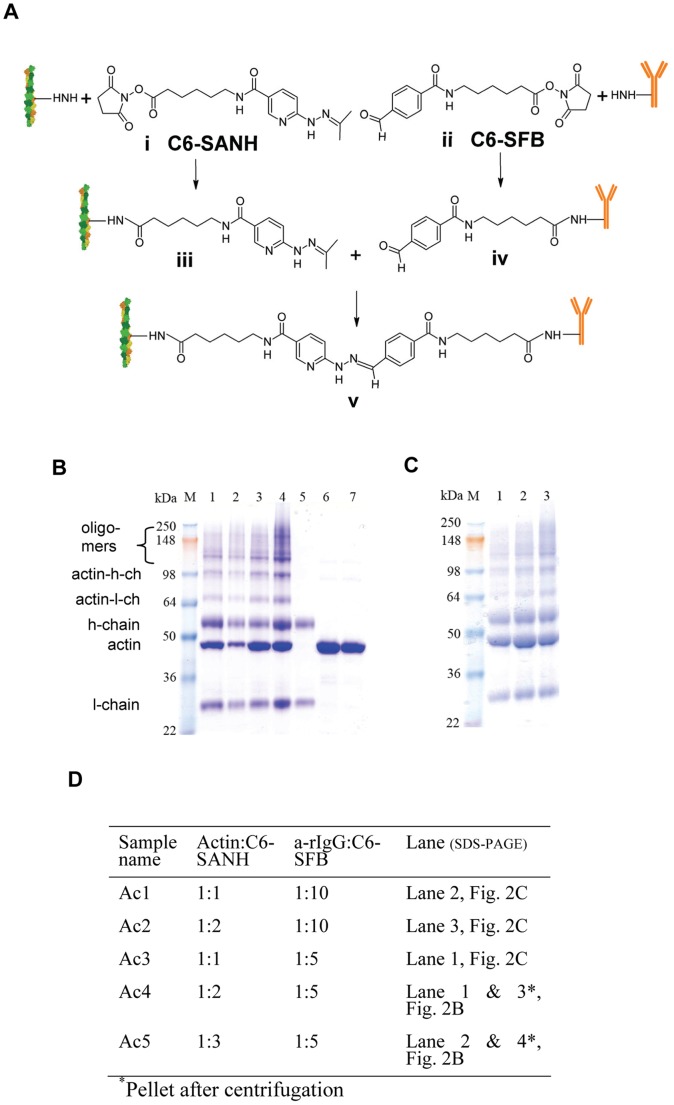
Figure 2. Covalent attachment of a-rIgG to actin monomer.
(A) Principle. C6-SANH (i, iii) aromatic hydrazine protected as acetone hydrazone and C6-SFB (ii, iv) reacted with primary amines of lysines on actin filament and a-rIgG, respectively. Next (v), aromatic hydrazone modified actin filament reacted with aromatic aldehyde modified a-rIgG forming stable bis-aryl hydrazone bond. (B) Gel under reducing conditions (SDS-PAGE) of actin-(a-rIgG) conjugates. Lanes 1–4: Conjugates Ac4 and Ac5 (2 and 3 molar excess of C6-SANH over actin, respectively, and 5 molar excess of C6-SFB over a-rIgG). Lanes 1–2 and 3–4 represent conjugates prior to ultracentrifugation and pellet after ultra-centrifugation (containing conjugated actin in filament form; F-actin), respectively. Lane 5. a-rIgG modified with 10 fold molar excess of C6-SFB. Lanes 6–7: non-conjugated actin reacted with 2 and 3 molar excess C6-SANH, respectively. The first lane denoted “M”: molecular marker (SeeBlue Plus2®). Approximately 3 µg of proteins in each well. (C) Lanes 1–3: conjugated samples Ac3, Ac1 and Ac2. Actin reacted with 1 (Ac3 and Ac1) and 2 (Ac2) molar excess of C6-SANH respectively. Labeling of different bands to the left: l-chain (l-ch) and h-chain (h-ch) denote antibody light chain and heavy chain, respectively. (D) Overview of samples shown in different lanes of SDS-PAGES in B and C.