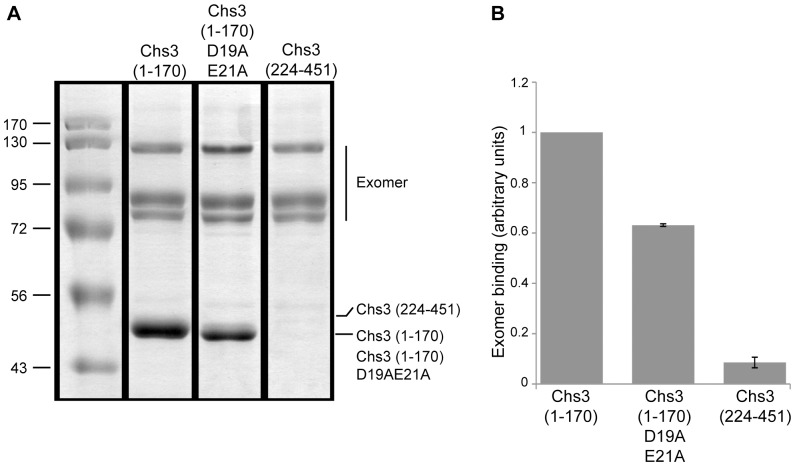
Figure 6. Mutation of D19 and E21 impairs the in vitro binding of Chs3p with exomer.
(A) Constructs corresponding to the full N-terminal cytosolic tail of Chs3p (Chs3 (1–170)), with and without the double substitution D19AE21A, or to the second cytosolic part of Chs3p (Chs3 (224–451)) were expressed in E. coli and purified as soluble GST-fusions. His-Chs5 and His-exomer complexes (expressed and purified from baculovirus infected culture cells [31]) were immobilized on Ni-NTA beads and incubated with 150 ug/ml purified GST-Chs3 fragments at RT for 30 min. Beads were washed and bound proteins were eluted with sample buffer followed by SDS-PAGE and Coomassie Blue staining. All samples shown were analyzed in the same gel. The images of each pertinent lane were cropped and reassembled. (B) The amount of each co-bound Chs3p fragment was quantified and normalized against the amount of Chs5p present in the same lane. The ratios of the amount of co-bound D19AE21A and 224–451 were normalized against the ratio of the wt pull-down, which was arbitrarily set to 1. The values from two independent experiments were averaged and graphed. Error bars represent standard deviations.