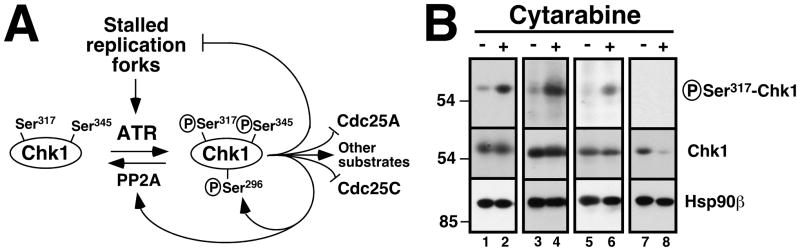
Figure 1. Effect of cytarabine on Chk1 phosphorylation in clinical AML samples in vivo.
A, schematic of the ATR/Chk1 pathway. In response to stalled replication forks, ATR catalyzes the activating phosphorylation of Chk1 on Ser317 and Ser345. Chk1 in turns phosphorylates a number of substrates, including Cdc25A (which contributes to S phase progression but is degraded after phosphorylation), Cdc25C (which contributes to G2/M progression but is inhibited by phosphorylation), substrates that stabilize replication forks, a substrate leading to phosphatase PP2A activation (which leads to Chk1 dephosphorylation), and Chk1 Ser296, an autophosphorylation site. B, marrow mononuclear cells were obtained from patients 6 (lanes 1, 2), 8 (lanes 3, 4), 12 (lanes 5, 6) and 2 (lanes 7, 8) in Cohort 1 before treatment (−) and after 48 h of single-agent cytarabine (+) at 400 mg/m2/day by continuous infusion. All samples shown contained >80% blasts (median 91%). Whole cell lysates were prepared from each fresh sample, subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antibodies that recognize the activating phosphorylation of Chk1 on Ser317 or total Chk1. Hsp90β served as a loading control.