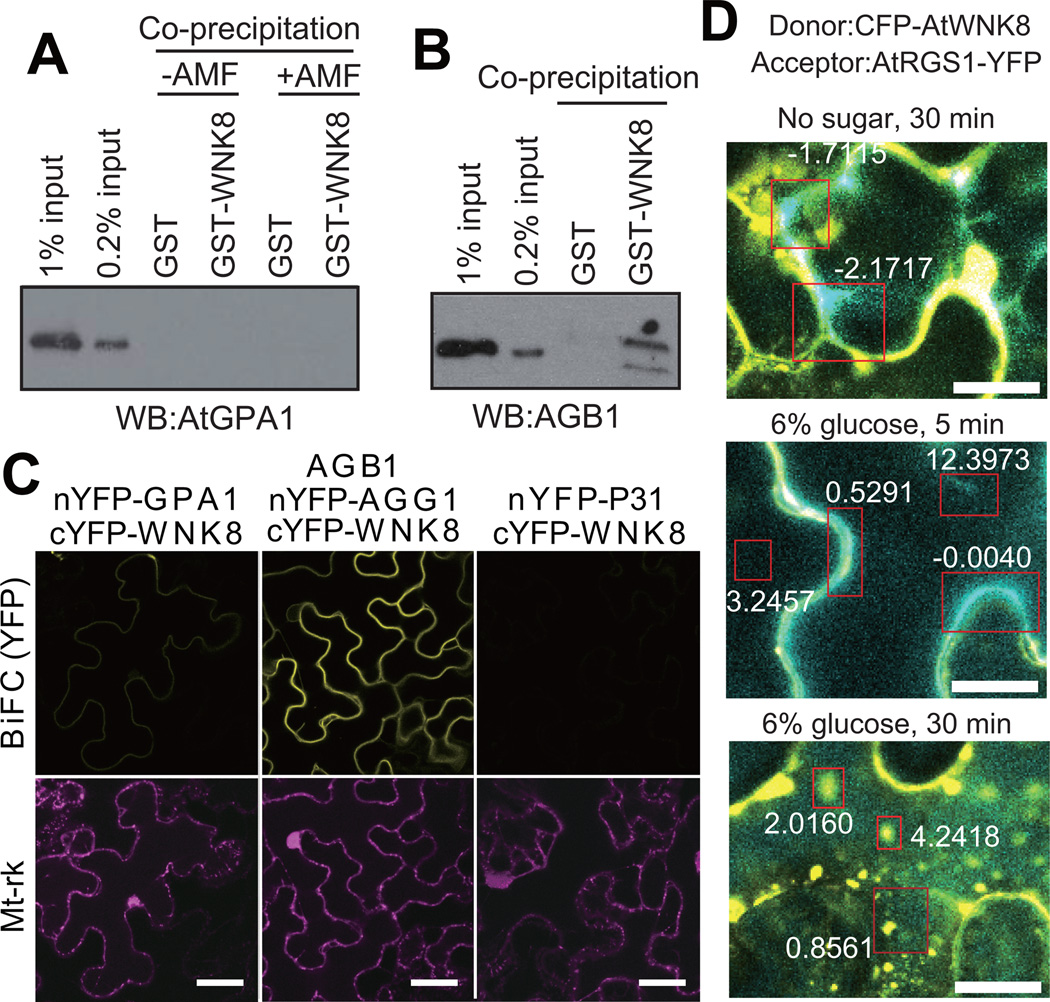
Figure 5. AtWNK8 physically interacts with the G-protein βγ subunit.
(A, B) In vitro binding between AtWNK8 and heterotrimeric G protein. Inactive, GDP-bound AtGPA1, AtGPA1 activated by aluminum tetrafluoride (AMF: 50 µM GDP, 30 µM AlCl3, 10 mM MgCl2 and 5 mM NaF) or AGB1/AGG1 was co-precipitated with GST or GST-AtWNK8. Proteins were subjected to immunoblot analysis with anti-AtGPA1 or anti-AGB1 antisera. The two different amounts of input protein (0.2% or 1% of total) were loaded as reference. (C) In vivo binding between AtWNK8 and heterotrimeric G protein. nYFP-tagged AtGPA1, AGG1 with AGB1, or P31 was co-transformed with cYFP-tagged AtWNK8 and mitochondrial marker (Mt-rk, transformation control) into tobacco leaves. Fluorescence complementation of split YFP and expression of RFP were observed by confocal fluorescence microscopy. Scale bars = 50 µm. (D) CFP-AtWNK8 associates with AtRGS1-YFP. Acceptor photobleaching of CFP-AtWNK8 and AtRGS1-YFP transiently expressed in tobacco in no sugar and 6% D-glucose for the indicated times. Bleached zones are in red boxes and numbers denote the net FRET value. WNK8 denotes AtWNK8. The method for determining the FRET efficiency indicated by the respective boxes is described in the Methods. Scale bars = 20 µm.